IMPACT CIRCLE
Join our Impact Circle for an exclusive look at the pioneering science happening at the Buck Institute. This unique program offers you the chance to meet our top scientists and dive into the forefront of aging research.
Each year, you'll hear pitches for "Blue Sky" projects—innovative ideas that are just starting and need initial funding to develop. These projects often involve collaborations that are too new for government grants but ripe with potential. Our scientists bring their most creative concepts, simplifying complex science into exciting, understandable projects just for our Circle Members.
Experience science in action and follow a research project for a year, seeing firsthand how your support brings brilliant ideas to life.
Donors join the Impact Circle by making a $5,000 contribution. If you have a Donor Advised Fund (DAF), recommending a grant to the Impact Circle is an easy and simple way to participate. Click here to find out more.
To learn more about the Impact Circle, contact Lisa Palma:
 |
Lisa Palma Director of Philanthropy |
Impact Circle Details
Scroll down to see the 2024 projects.
Cargo Delivery to the Brain
The Potential Impact: Our protein-based technology provides a new method to deliver purified proteins and potentially other types of therapeutics including antisense oligonucleotides, siRNAs, viral vectors, and nanoparticles to the brain.
Buck Collaborators:
 Dr. Lisa Ellerby |
 Dr. Barbara Bailus |
What is the impact of the Impact Circle? Our donors come back, year after year, anxious to learn and participate. And our scientists are raring to go every year because, it turns out that the Impact Circle has provided a lot of bang for the Buck scientist.
Up-ending the Scientific Status Quo

Dr. Judith Campisi
The first winners of the Impact Circle, Judy Campisi and Julie Andersen, used their funding to carve out entirely new scientific territory. Campisi is the world’s leading authority on cellular senescence – the process by which cells cease to divide, then accumulate and secrete toxins – and its role in aging. Yet she had never considered that cellular senescence might happen in the brain because most neurons do not divide to begin with. Andersen, a neuroscientist renowned for her expertise in Parkinson’s disease (PD), proposed that they investigate whether cellular senescence occurs in the brain and, if so, whether it is linked to PD.

Dr. Julie Andersen
“We had submitted grants on the topic to the NIH and got rejected again and again,” recalls Andersen. “I assume it was because people in the aging field knew of cellular senescence, but people in neuroscience had no idea what it was.” Now they do, thanks, in part, to the Impact Circle. Andersen and Campisi were able to initiate experiments with the money from the Impact Circle. The results from those experiments led to more funding from the NIH and then from the Michael J. Fox Foundation. “Basically, all that work ended up in Cell Reports in January of 2018 as the first paper to show that if you knocked out senescent cells in the brain, it prevented an age-related neurodegenerative disease – Parkinson’s,” says Andersen.
The Gift That Goes on Giving
 Likewise, Pankaj Kapahi was recently awarded a National Institutes of Health (NIH) RO1 grant, one of the institute’s largest and most prestigious, for a study that combines all three of his Circle projects. With the grant, Kapahi is exploring why people with diabetes are at two times the risk of Alzheimer’s disease. He hypothesizes that the accumulation of advance glycation end products (AGEs) – toxic byproducts of glucose metabolism that are more prevalent in people with diabetes – are to blame for the neurodegeneration that develops.
Likewise, Pankaj Kapahi was recently awarded a National Institutes of Health (NIH) RO1 grant, one of the institute’s largest and most prestigious, for a study that combines all three of his Circle projects. With the grant, Kapahi is exploring why people with diabetes are at two times the risk of Alzheimer’s disease. He hypothesizes that the accumulation of advance glycation end products (AGEs) – toxic byproducts of glucose metabolism that are more prevalent in people with diabetes – are to blame for the neurodegeneration that develops.
“The Circle supported the preliminary work it took to get NIH funding,” says Kapahi. “Our NIH grant wouldn’t have been possible without it.” Circle funding has had a fundamental impact on his career. “The Impact Circle pushed my lab in a whole new direction,” adds Kapahi. Prior to his wins, his lab was focused primarily on dietary restriction. “This seed funding has helped us create more far-reaching connections with diseases like Parkinson’s,” he says.
Building Community

Our Impact Circle, like all circles, is ongoing. Winners continually apprise Circle Members of their progress. Indeed, six months after Campisi and Andersen won, they got together with Circle members to celebrate and share their work. “It’s so nice to be able to interact with people on a personal level about your work,” says Andersen. “The Circle Members have been so supportive and it is really so nice knowing that they believe in us.”
Previous Winners:
2023 Lisa Ellerby and Barbara Bailus
2022 John Newman and Brianna Stubbs
2021 Tara Tracy and Kai Zhou
2020 Dan Winer and David Furman
2019 Simon Melov and Nicolas Martin
2018 Pankaj Kapahi and Neelanjan Bose
2017 Jennifer Garrison and Birgit Schilling
2017 Pankaj Kapahi and John Newman
2016 Julie Andersen and Pankaj Kapahi
2015 Dale Bredesen and Brian Kennedy
2014 Judy Campisi and Julie Andersen
Blood brain barrier: The blood–brain barrier is a highly selective semipermeable border of endothelial cells that prevents toxins in the circulating blood from crossing into brain.
Glycation (sometimes called non-enzymatic glycosylation) is the attachment of a sugar to a protein or lipid. Typical sugars that participate in glycation are glucose, fructose, or their derivatives. Glycation is a biomarker for diabetes and is implicated in some diseases and in aging.
Human induced pluripotent stem (iPS) cells: are derived from skin or blood cells that have been reprogrammed back into an embryonic-like, undifferentiated state that enables the development of any type of human cell needed for therapeutic purposes. Shinya Yamanaka of the Gladstone Institute and UCSF, received a Nobel for discovering them. They have transformed biological research. iPS cells can be developed into organoids, which are miniaturized and simplified version of an organ produced in vitro in three dimensions that show realistic micro-anatomy. Organoids made from human tissues allow scientists to study the effectiveness of novel therapeutics in a safe, inobtrusive and precise way.
Intergenerational – existing or occurring between generations. Example: Research has shown the impact of polychlorinated biphenyls (PCBs) – chemicals that were used in electrical equipment starting in the 1920’s – across generations. Although they have been phased out of production, PCBs are still being released into the environment from preexisting applications and improper disposal of products that contain them. Because of their chemical stability and transportability, they accumulate in the food chain, enter our food supply, and pass from mother to infant in utero and during breastfeeding, with potentially harmful effects on the endocrine, immune, nervous, and reproductive systems.
Synapse – the space between two neurons where chemical substances are retained to transfer electrical signals from one neuron to another.
T-cells – is an immune cell, made in the bone marrow, that attacks virus-infected cells, foreign cells and cancer cells. The T in T-cells stands for thymus, an organ at the front of the trachea, where T-cells mature.
Tau – is a protein shaped like a tube that transports nutrients from one nerve cell to another. Damaged and misshapen tau are found in multiple forms of brain disease, including Alzheimer’s disease, chronic traumatic encephalopathy, Pick disease, frontotemporal dementia with parkinsonism-17, progressive supranuclear Palsy, and corticobasal degeneration.
Lisa Palma
Director, Office of Philanthropy
LPalma@buckinstitute.org
415-2092027
2024 PROJECTS
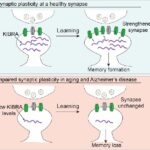
Testing if a new synthetic peptide can restore memory formation in aging and in Alzheimer's disease
New clinical trial testing the effect of a special formulation designed to slow aging and improve healthspan
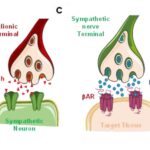
Discovering how to “tune” the sympathetic nervous system to reduce the harmful effects of chronic stress