KAPAHI LAB
Lab focus
Dietary restriction (DR), the reduction in nutrient intake without malnutrition, has been well documented as a means to extend lifespan and slow age-related diseases in many systems. We and others have previously demonstrated that lifespan extension by inhibition of the TOR pathway overlaps with the effects of DR in D. melanogaster, S. cerevisiae, and C. elegans. However, other DR response mechanisms exist that remain undiscovered. The overall goal of the Kapahi lab is to understand how an organism responds to nutrient status to influence health and disease.
We utilize worms, flies, and mice as model systems to understand how nutrients influence age-related changes in specific tissues and disease processes. We take creative approaches to develop models for various human diseases that are influenced by nutrient status using invertebrates. We study how various physiological and molecular processes, including fat metabolism, circadian clocks, advanced glycation end products, calcification, and intestinal permeability, are influenced by nutrients to impact organismal health and survival. We collaborate with multiple groups at University of California, San Francisco, and University of California, Berkeley, to undertake interdisciplinary approaches to translate our findings from multiple models to humans.
Why it matters
Our work has relevance to certain age-related human diseases, including diabetes, Alzheimer’s disease, kidney stone formation, intestinal diseases, and obesity. There is an ongoing debate about the limitation of lifespan as a measure of aging and the need to assess healthspan to find the most promising interventions for humans. Through functional measures of different tissue functions and disease models, we are also examining the relationship between healthspan and lifespan.
Our work on aging in simple animals has led to real possibilities of treating human diseases, something I never thought possible when I began my career.
Pankaj Kapahi, PhD
CENTER DETAILS
Dr. Kapahi received his PhD from the University of Manchester, where he worked with Tom Kirkwood. He did his postdoctoral work with Seymour Benzer at Caltech and Michael Karin at University of California, San Diego. He joined the Buck Institute as an assistant professor in 2004.
Dr. Kapahi has published more than 80 scientific papers and holds three current patents. He has been recognized for his scientific excellence with many awards, including the Eureka Award from the National Institute on Aging, a New Scholar Award from the Ellison Medical Foundation, a Glenn Award for Research in Biological Mechanisms of Aging, the Nathan Shock Young Investigator Award, and the Breakthrough in Gerontology and Julie Martin Mid-career awards from AFAR. He currently serves on the editorial board of Aging Cell, Aging, and PLOS Genetics. Dr. Kapahi also initiated the first master’s degree course in gerontology at the Buck Institute.
-
 Sudipta Bar Postdoctoral Researcher
Sudipta Bar Postdoctoral ResearcherDr. Bar joined the Kapahi lab as a postdoctoral research fellow in July 2018. He received his Ph.D. in Biological Sciences from Indian Institute of Science Education and Research Kolkata, India where he studied lysosomal storage disorders using Drosophila and cell culture. At Buck, he utilizes Drosophila genetics, molecular biology, and bioinformatics tools to understand the environmental and genetic factors responsible for neurodegeneration in Alzheimer’s and related diseases. He recently received the Larry L. Hillblom postdoctoral fellowship grant (2019).
SBar@buckinstitute.org
-
 Lizbeth Enriquez Lab Manager / Research Associate
Lizbeth Enriquez Lab Manager / Research AssociateLizbeth is a graduate student who joined the Kapahi Lab in 2021. She attended Dominican University of California where she received her bachelor's degree in molecular biology. Her research at the Buck Institute aims to understand the function of advanced glycation end products (AGEs) in aging and age-related disorders using in vivo mammalian model systems.
LEnriquez@buckinstitute.org
-
 Lindsay Gann Dominican University Graduate Student
Lindsay Gann Dominican University Graduate StudentLindsay is a master’s student at Dominican University of California. She received a bachelor’s degree in biochemistry with a minor in anthropology from Arizona State University, as well as a bachelor’s degree in English from Trinity International University. Lindsay’s research is focused on understanding the role of cellular senescence in age-related conditions such as menopause and obesity using the drosophila model organism.
LGann@buckinstitute.org
-
 Myla Gupta Dominican University Graduate Student
Myla Gupta Dominican University Graduate StudentMyla is a graduate student at Dominican University pursuing an M.S. in biological sciences. She recently graduated from UCSB with a B.S. in biological sciences, where she worked as a research assistant in the Simpson Lab studying the neural mechanics of Drosophila motor coordination. This undergrad research developed her curiosity for neuroscience and led Myla to her current projects in the Kapahi Lab, where she is investigating neural expression of genes that drive aging in a Drosophila model. She hopes to contribute to research in neurodegeneration to help ease the chronic suffering that often accompanies age."
MGupta@buckinstitute.org
-
 Worthy Gutierrez Dominican University Graduate Student
Worthy Gutierrez Dominican University Graduate StudentWGutierrez@buckinstitute.org
-
 Kiyomi Kaneshiro, PhD Postdoctoral Researcher
Kiyomi Kaneshiro, PhD Postdoctoral ResearcherKiyomi joined the Kapahi lab as a postdoctoral research fellow under the co-mentorship of Judith Campisi in 2021. She received her PhD from UC Santa Cruz where her research focused on understanding epigenetic inheritance using the model organism C. elegans. Her research at the Buck Institute, aims to understand the role of advanced glycation end products (AGEs) in cellular senescence and age-related neurodegenerative diseases using in vitro and in vivo mammalian model systems.
KKaneshiro@buckinstitute.org
-
 Vikram Narayan, PhD Postdoctoral Researcher
Vikram Narayan, PhD Postdoctoral ResearcherVikram joined the Kapahi lab as a postdoctoral research fellow in 2022. He received his joint PhD from The University of Queensland (Australia) and the University of Exeter (UK) where his research focused on genetic and environmental drivers of aging and other life-history traits using the model organism Drosophila and human genomic data. Using multidisciplinary approaches, he is interested in exploring how gene-environment interactions govern health and lifespan. His research at the Buck Institute, aims to bridge the analysis of model-organism functional data, evolutionary biology, and human genomics to better understand the biology of aging and aging-related diseases.
vnarayan@buckinstitute.org
-
 Johnathan Rylee, PhD Postdoctoral Researcher
Johnathan Rylee, PhD Postdoctoral ResearcherJohnathan joined the Kapahi lab as a postdoc in March 2024. He received his PhD from Indiana University where he studied the genetics of photoreceptor differentiation and disease using Drosophila and zebrafish. He is interested in eye aging, and how photoreceptor lipid profiles are maintained throughout their lifespan.
JRylee@buckinsitute.org
-
 Muniesh Muthaiyan Shanmugam , PhD Postdoctoral Researcher
Muniesh Muthaiyan Shanmugam , PhD Postdoctoral ResearcherMuniesh is interested in understanding adult-onset disease mechanisms with a specific focus on neurodegeneration and aging. He did his doctoral research on the regulation of synaptic vesicles’ fast axonal transport in neurons of C. elegans at National Tsing Hua University (Taiwan). He is currently studying the role of Advanced Glycation End-products (AGEs) in accelerating aging and neurodegeneration in C. elegans. Muniesh’s other interests include development of C. elegans models for neurodegenerative diseases, understanding exercise physiology and nutrition.
mshanmugam@buckinstitute.org
-
 Parminder Singh, PhD Postdoctoral Researcher
Parminder Singh, PhD Postdoctoral ResearcherDr. Singh Joined Dr. Kapahi's lab as a Postdoctoral Research Fellow in the summer of 2021. He received his PhD from the National Institute of Immunology, Delhi, India. His research at the Buck Institute aims to explore the role of advanced glycation end products (AGEs) in mitochondrial disorder and addiction. He is a very affable person and spends his free time exploring and cooking spicy food.
PSingh@buckinstitute.org
-
 Vineeta Tanwar Research Scientist / Project Manager
Vineeta Tanwar Research Scientist / Project ManagerVineeta joined the Kapahi lab as a Senior Researcher in 2022. She received her PhD from All India Institute of Medical Sciences, New Delhi, India. Her postdoctoral work at Vanderbilt University School of Medicine exploited human and mouse embryonic stem cells to optimize cardiac repair and regeneration therapy. Her continued research work at The Ohio State University in the field of Environmental Cardiology allowed her to investigate external triggers of cardiac disease, with emphasis on particulate matter exposure. She also established collaboration with United States Department of Veterans Affairs to investigate the cardio-pulmonary effects of World Trade Center (WTC) dust exposure. In her recent science industry-oriented research conducted at the Center for Integration of Science and Industry, Bentley University, she focused on quantitative measure of basic science innovation in the FDA approved drugs in the past decade with a goal to obtain more informative insight into the drug innovation trend and on health values created by biopharmaceutical companies. She is passionate about science journalism and enjoys communicating science to non-scientific audience to raise scientific awareness and fight scientific misinformation.
VTanwar@buckinstitute.org
-
 Dipti Verma, PhD Postdoctoral Researcher
Dipti Verma, PhD Postdoctoral ResearcherDipti joined the Kapahi lab as a postdoctoral researcher in July 2024. She received her PhD from the Department of Molecular and Human Genetics, Banaras Hindu University, India where she studied the functional implications of Non-muscle myosin II Zipper in the regulation of Notch signaling in Drosophila. Her research at the Buck Institute is focused on studying the molecular mechanisms behind delayed ovarian senescence and longevity upon dietary restriction.
DVerma@buckinstitute.org
-
 Kenneth Wilson, PhD Postdoctoral Researcher
Kenneth Wilson, PhD Postdoctoral ResearcherKenneth received his bachelor’s degree in molecular and cell biology from University of California Berkeley, his master’s degree in biological sciences from Dominican University of California and his PhD from University of Southern California. His current research focuses on understanding how natural genetic variation can influence response to diet to affect longevity and health.
KAWilson@buckinstitute.org
-
 Yifan Xiang, MD Postdoctoral Researcher
Yifan Xiang, MD Postdoctoral ResearcherYifan joined the Kapahi Lab as a Postdoctoral Researcher in 2023. She received her MD from the Zhongshan Ophthalmic Center, Sun Yat-sen University. Her research at the Buck aims to develop prediction models for aging and age-related diseases with AI.
YXiang@buckinstitute.org
-
 Zhixin (Alice) Zhang Research Associate and Lab Manager
Zhixin (Alice) Zhang Research Associate and Lab ManagerAlice received her B.S. in bioengineering from UC Berkeley in 2022 with a concentration in cell and tissue engineering. While at Berkeley, Alice studied muscle aging and rejuvenation in Dr. Irina Conboy’s laboratory, which propelled her towards the field of aging research. She joined the Campisi lab as a research associate in May 2023.
zzhang@buckinstitute.org
Kapahi lab protocols: https://en.bio-protocol.org/labinfo.aspx?labid=8
- Investigating the Role of Advanced Glycation End (AGE) products in aging, diabetes, and neurodegeneration
Diabetes is a metabolic disease resulting from elevated glucose over a prolonged period. In the United States, 11.3 percent of all adults 20 or over have diabetes. Diabetes overall at least doubles the risk of death. Diabetes leads to various complications and organ failure, including cardiovascular diseases, kidney failure, diabetic retinopathy in the United States in 2010. Diabetes also more than doubles the risk of Alzheimer’s and Parkinson’s disease. However, the mechanisms by which diabetes causes these pathologies remain poorly understood
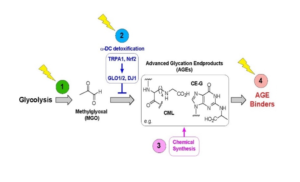
Fig. 1. Biochemical model showing points of intervention to decrease the neurodegeneration-inducing effects of MGO and AGEs. The balance between production of MGO from glycolysis and detoxification determines the steady-state level of MGO, which in turn drives the rate of AGE formation. AGEs promote neurodegeneration and aging. Numbers 1–4 represent sites of interventions designed to ameliorate AGE-mediated toxicity.
Our working hypothesis is that prolonged elevation of glucose leads to glycation of macromolecules like protein and lipids, by forming Advanced Glycation End-products (AGEs). Methylglyoxal (MGO) and other glyoxals, which are unavoidable byproducts of anaerobic glycolysis and lipid peroxidation, react indiscriminately with proteins, lipids, and DNA to yield a heterogeneous array of molecules collectively called AGEs The formation of AGEs damages cellular macromolecules and has been associated with the complications of diabetes and other diseases. We have developed a model to study various pathologies including diabetic neuropathy, neurotoxicity, and accelerated aging using C. elegans within a two-week timeframe. We have established a C. elegans model, based on impaired glyoxalases (GLO1 or DJ-1), to broadly study MGO related stress. We show that, in comparison to wild-type animal, the mutants rapidly exhibit several pathogenic phenotypes, including hyperesthesia, neuronal damage, reduced motility, and early mortality. We further demonstrate TRPA-1/TRPA1 as a sensor for a-DCs, conserved between worms and mammals. Moreover, TRPA-1 activates SKN-1/Nrf via calcium-modulated kinase signaling, ultimately regulating the glutathione-dependent (GLO1) and co-factor-independent (DJ1) glyoxalases to detoxify a-DCs. Using our model, we are uncovering genetic and pharmacological targets that modulate the onset of AGEs pathology in our worm model at different steps shown in Figure 1. We are currently pursuing these targets in various models of neurodegenerative diseases using worms, mice, and induced pluripotent stem cells.
- The role of diet and circadian clocks in aging and neurodegeneration
Dietary Restriction (DR) is the reduction of particular or total nutrient intake without causing malnutrition. DR extends lifespan and delays the onset of age-related neurodegeneration in models of neurodegenerative disease in diverse organisms, including yeast, flies, and rodents. Several studies in mice have documented that caloric restriction or time-restricted feeding slow age-related neurological decline in both normal aging and models of AD. However, the molecular mechanisms through which nutrient restriction protects the brain and other tissues during normal aging and AD are just beginning to be elucidated.
We propose to use the fly to study this question as it is a well-established and expedient model to study aging, dietary changes, and neurodegenerative diseases. We are also studying the cross-talk between nutrient-sensing pathways and circadian clocks to modulate aging and neurodegeneration. Our interdisciplinary studies involve the use of genetics, proteomics, bioinformatics to define the nutrient responsive pathways that modulate aging and neurodegenration. Furthermore, we are using flies, mice and induced pluripotent stem cells to study pathways that influence aging and neurodegeneration.
LAB GALLERY
Selected Publications
-
KA Wilson, M Chamoli, TA Hilsabeck, M Pandey, S Bansal, G Chawla, P Kapahi. Evaluating the beneficial effects of dietary restrictions: A framework for precision nutrigeroscience, Cell Metabolism – In Press
-
Wilson KA, Beck JN, Nelson CS, Hilsabeck TA, Promislow D, Brem RB, Kapahi P GWAS for Lifespan and Decline in Climbing Ability in Flies upon Dietary Restriction Reveal decima as a Mediator of Insulin-like Peptide Production. Curr Biol. 2020 Jul 20;30(14):2749-2760
- Jyotiska Chaudhuri, Yasmin Bains, Sanjib Guha, Arnold Kahn,David Hall, Neelanjan Bose, Alejandro Gugliucci, Pankaj Kapahi, The Role of Advanced Glycation End Products in Aging and Metabolic Diseases: Bridging Association and Causality, Cell Metabolism, Volume 28, Issue 3, 4 September 2018, Pages 337-352
- Zee, T., Bose, N., Stoller, M., Kapahi, P. Alpha-lipoic acid ameliorates stone formation in a mouse model of cystinuria. (Accepted at Nature Medicine).
- Luis, N. M., Wang, L., Ortega, M., Deng, H., Katewa, S. D., Li, P. W., Karpac, J., Jasper, H., Kapahi, P. Intestinal IRE1 is required for increased triglyceride metabolism and longer lifespan under dietary restriction. Cell Rep, 25, 17(5), 1207–1216.
- Chaudhuri, J., Bose, N., Gong, J., Hall, D., Rifkind, A., Bhaumik, D., Peiris, T. H., Chamoli, M., Le, C. H., Liu, J., Lithgow, G. J., Ramanathan, A., Xu, X. Z., Kapahi, P. (2016 Nov 21). A Caenorhabditis elegans model elucidates a conserved role for TRPA1-Nrf signaling in reactive α-Dicarbonyl detoxification. Curr Biol, 26(22), 3014–25.
- Katewa, S. D., Akagi, K., Bose, N., Rakshit, K., Camarella, T., Zheng, X., Hall, D., Davis, S., Nelson, C. S., Brem, R. B., Ramanathan, A., Sehgal, A., Giebultowicz, J. M., Kapahi, P. (2016 Jan 12). Peripheral clocks modulate lifespan and fat metabolism upon dietary restriction. Cell Metab, 23(1), 143-54. doi: 10.1016/j.cmet.2015.10.014. Epub 2015 Nov 25.
- Chen, D., Li, P. W., Goldstein, B. A., Cai, W., Thomas, E. L., Chen, F., Hubbard, A. E., Melov, S., Kapahi, P. (2013). Germline signaling mediates the synergistically prolonged longevity produced by double mutations in daf-2 and rsks-1 in C. elegans. Cell Rep, 5, 1600–10.
- Katewa, D., Demontis, F., Kolipinski, M., Hubbard, A., Gill, M., Perrimon, N., Melov, S., Kapahi, P. (2012). Intra-myocellular triglyceride turnover plays a critical role in mediating responses to dietary restriction in Drosophila melanogaster. Cell Metab, 16, 97–103.
- Zid, B. M., Rogers, A. N., Katewa, S. D., Vargas M. A., Kolipinski, M. C., Lu, T. A., Kapahi, P. (2009). 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell, 139, 149–60.
- Pan, K. Z., Palter, J. E., Rogers, A. N., Olsen, A., Chen, D., Lithgow, G. J., Kapahi, P. Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell, 6(1), 111–9. PMID: 17266680.
- Kapahi, P., Zid, B. M., Harper, T., Koslover, D., Sapin, V., Benzer, S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol, 14, 885–90.
- Rossi, A., Kapahi, P., Natoli, G., Takahashi, T., Chen, Y., Karin, M. (2000). Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature, 403(6765), 103–8.
Dr. Kapahi’s full publication list
IN THE NEWS





















