GERENCSER LAB
Lab focus
The Gerencser lab studies the bioenergetic function of mitochondria. We seek to understand why and how mitochondria lose fine-tuning in aging and age-related disease. The primary focus of the lab is type 2 diabetes. Mitochondrial energetics play a critical role in type 2 diabetes, affecting insulin secretion in pancreatic β-cells, and in the development of insulin resistance in peripheral tissues. The overall goal of the lab is to uncover cellular mechanisms that could be treatment targets for preventing functional impairment of β-cells and the development of type 2 diabetes.
Mitochondrial dysfunction (collectively referring to abnormalities in mitochondrial metabolism, ATP synthesis, reactive oxygen production, protein and solute import, quality control, biosynthetic pathways and retrograde signaling) contributes to a host of age-related diseases, and possibly to the physiology of normal aging. Chronically developing dysfunction may be caused by subtle deviations from the normal, or such abnormalities may affect only small sub-populations of cells in a tissue.
To explore such dysfunction, we have developed sensitive, quantitative assays of mitochondrial function. Continued technology development and multimodal detection is a focus of the lab. We use state-of-the-art technology, including multiphoton microscopy, super resolution microscopy and high-content microscopy. We develop image analysis using deep learning for quantification of microscopy image data. We also combine live-cell functional imaging with single-cell omics and CRISPR KO.
Why it matters
On average, people diagnosed with type 2 diabetes lose 6 years of life expectancy compared to healthy individuals. Understanding basic mechanisms of the disease are expected to support nutraceutical and/or pharmacological interventions to improve blood glucose control. Our work is also relevant to mitigating age-related loss of muscle function and bone density, both of which contribute to frailty.
Aging, which often features progressive dysfunction, is likely perpetuated by subtle deviations from normal function. Our aim is to track such subtleties with the overarching goal of intervening in the aging process.
Akos Gerencser, MD, PhD
LAB DETAILS
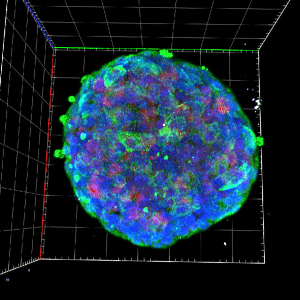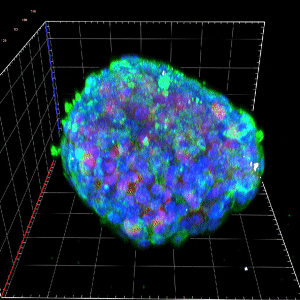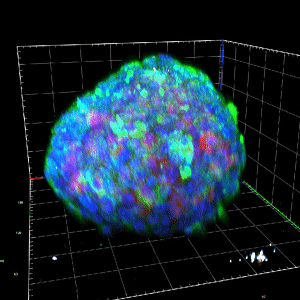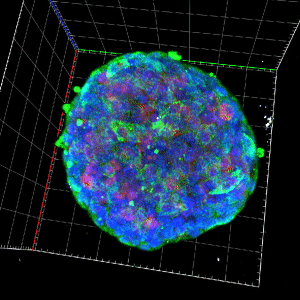
The mitochondrial control of insulin secretion in type 2 diabetes
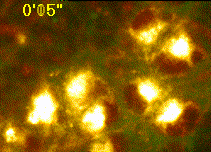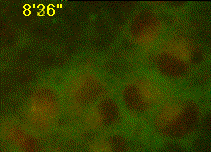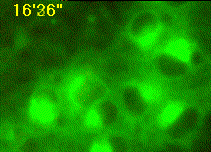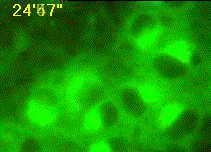
Developing assays to quantify function in cells and tissues
Dr. Gerencser is a research associate professor and the director of the Morphology and Imaging (microscopy) Core at the Buck Institute. Dr. Gerencser received his MD and PhD from Semmelweis University, Hungary. He received an additional MSc in Biomedical Engineering at Budapest University of Technology and Economics, Hungary. He did his postdoctoral work at Sanford Burnham, La Jolla, before coming to the Buck in 2007.
Dr. Gerencser has over 50 scientific publications, which have been cited more than 5000 times. He has over two decades of experience in mitochondrial and cell physiology, including fields highly relevant to geroscience: cellular senescence, neurodegenerative diseases, stem cell biology, bone quality, and type 2 diabetes. His dual background in medicine and engineering allows an interdisciplinary vantage point in biomedical sciences, ranging from understanding human disease conditions to designing technology-driven approaches to resolve and better understand functional decline with age.
Dr. Gerencser’s research accomplishments include identifying a novel mechanism in mitochondrial regulation of insulin secretion in pancreatic β-cells1-3. He developed and contributed to the development of platforms to quantify bioenergetics and other functional properties of cellular systems. These include development of the unbiased, absolute millivolts determination of mitochondrial membrane potential in monolayer cells or 3D tissue cultures and explants4-6. He has contributed to the now widely used Seahorse Extracellular Flux Analyzer (then Seahorse Bioscience, now Agilent) by discovering how oxygen diffusion is distorting the sensor readout and developing a computational correction for it7. He has contributed to the development of selective small-molecule suppressors of superoxide formation at the complex I of the mitochondrial electron transport chain, known as S1QELs8, and he discovered that complex I Q-site is a source of superoxide during normal, forward electron transport in intact cells8,9. Furthermore, he has also contributed to elucidating the roles of mitochondria and intracellular calcium in aspects of neurodegenerative diseases10-15, stem cells16-19, and cellular senescence20 in numerous collaborative works, including the fields of Alzheimer’s21,22, Parkinson’s18,23, and Huntington’s24-26 diseases. He is a contributor to the SenNet Consortium mapping cellular senescence in healthy human aging27.
Dr. Gerencser is the founder of Image Analyst Software and developed Image Analyst MKII, a microscopy image analysis tool enabling analysis of multiplexed image data in e.g. mitochondria and cellular senescence-related paradigms.
References
- Gerencser, A. A. Bioenergetic Analysis of Single Pancreatic β-Cells Indicates an Impaired Metabolic Signature in Type 2 Diabetic Subjects. Endocrinology 156, 3496-3503 (2015). https://doi.org/10.1210/en.2015-1552
- Gerencser, A. A., Mookerjee, S. A., Jastroch, M. & Brand, M. D. Positive Feedback Amplifies the Response of Mitochondrial Membrane Potential to Glucose Concentration in Clonal Pancreatic Beta Cells. Biochimica et Biophysica Acta – Molecular Basis of Disease 1863, 1054-1065 (2017). https://doi.org/10.1016/j.bbadis.2016.10.015
- Gerencser, A. A. Metabolic activation-driven mitochondrial hyperpolarization predicts insulin secretion in human pancreatic beta-cells. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1859, 817-828 (2018). https://doi.org/10.1016/j.bbabio.2018.06.006
- Gerencser, A. A. et al. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. The Journal of physiology 590, 2845-2871 (2012). https://doi.org/10.1113/jphysiol.2012.228387
- Gerencser, A. A., Mookerjee, S. A., Jastroch, M. & Brand, M. D. Measurement of the Absolute Magnitude and Time Courses of Mitochondrial Membrane Potential in Primary and Clonal Pancreatic Beta-Cells. PloS one 11, e0159199 (2016). https://doi.org/10.1371/journal.pone.0159199
- Lerner, C. A. & Gerencser, A. A. Unbiased Millivolts Assay of Mitochondrial Membrane Potential in Intact Cells. Methods Mol Biol 2497, 11-61 (2022). https://doi.org/10.1007/978-1-0716-2309-1_2
- Gerencser, A. A. et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Analytical chemistry 81, 6868-6878 (2009). https://doi.org/10.1021/ac900881z
- Brand, M. D. et al. Suppressors of Superoxide-H2O2 Production at Site IQ of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab 24, 582-592 (2016). https://doi.org/10.1016/j.cmet.2016.08.012
- Gibbs, E. T. et al. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem J 480, 363-384 (2023). https://doi.org/10.1042/BCJ20220611
- Chinopoulos, C., Gerencser, A. A., Doczi, J., Fiskum, G. & Adam-Vizi, V. Inhibition of glutamate-induced delayed calcium deregulation by 2-APB and La3+ in cultured cortical neurones. Journal of neurochemistry 91, 471-483 (2004). https://doi.org/10.1111/j.1471-4159.2004.02732.x
- Gerencser, A. A., Doczi, J., Töröcsik, B., Bossy-Wetzel, E. & Adam-Vizi, V. Mitochondrial swelling measurement in situ by optimized spatial filtering: astrocyte-neuron differences. Biophysical journal 95, 2583-2598 (2008). https://doi.org/10.1529/biophysj.107.118620
- Choi, S., Gerencser, A. A. & Nicholls, D. G. Bioenergetic Analysis of Isolated Cerebrocortical Nerve Terminals on a Microgram Scale: Spare Respiratory Capacity and Stochastic Mitochondrial Failure. Journal of Neurochemistry 109, 1179-1191 (2009). https://doi.org/10.1111/j.1471-4159.2009.06055.x
- Gerencser, A. A. et al. Real-time visualization of cytoplasmic calpain activation and calcium deregulation in acute glutamate excitotoxicity. Journal of neurochemistry 110, 990-1004 (2009). https://doi.org/10.1111/j.1471-4159.2009.06194.x
- Flynn, J. M. et al. Impaired spare respiratory capacity in cortical synaptosomes from Sod2 null mice. Free radical biology & medicine 50, 866-873 (2011). https://doi.org/10.1016/j.freeradbiomed.2010.12.030
- Kushnareva, Y. E. et al. Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell death and differentiation 20, 353-365 (2012). https://doi.org/10.1038/cdd.2012.128
- Birket, M. J. et al. A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. Journal of cell science 124, 348-358 (2011). https://doi.org/10.1242/jcs.072272
- Deng, H., Gerencser, A. A. & Jasper, H. Signal integration by Ca 2+ regulates intestinal stem-cell activity. Nature 528, 212-217 (2015). https://doi.org/10.1038/nature16170
- Shaltouki, A. et al. Mitochondrial alterations by PARKIN in dopaminergic neurons using PARK2 patient-specific and PARK2 knockout isogenic iPSC lines. Stem Cell Reports 4, 847-859 (2015). https://doi.org/10.1016/j.stemcr.2015.02.019
- Guntur, A. R. et al. Osteoblast-like MC3T3-E1 Cells Prefer Glycolysis for ATP Production but Adipocyte-like 3T3-L1 Cells Prefer Oxidative Phosphorylation. Journal of Bone and Mineral Research 33, 1052-1065 (2018). https://doi.org/10.1002/jbmr.3390
- Wiley, C. D. et al. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabolism 23, 303-314 (2016). https://doi.org/10.1016/j.cmet.2015.11.011 , eprint = 9605103
- Barsoum, M. J. et al. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J 25, 3900-3911 (2006). https://doi.org/10.1038/sj.emboj.7601253
- Choi, S. W. et al. No Consistent Bioenergetic Defects in Presynaptic Nerve Terminals Isolated from Mouse Models of Alzheimer’s Disease. Journal of Neuroscience 32, 16775-16784 (2012). https://doi.org/10.1523/JNEUROSCI.2414-12.2012
- Choi, S. W. et al. Intrinsic bioenergetic properties and stress sensitivity of dopaminergic synaptosomes. The Journal of neuroscience : the official journal of the Society for Neuroscience 31, 4524-4534 (2011). https://doi.org/10.1523/JNEUROSCI.5817-10.2011
- Peters, T. W. et al. Natural Genetic Variation in Yeast Reveals That NEDD4 Is a Conserved Modifier of Mutant Polyglutamine Aggregation. G3 (Bethesda, Md.) 8, 3421-3431 (2018). https://doi.org/10.1534/g3.118.200289
- Song, S. et al. Postnatal Conditional Deletion of Bcl11b in Striatal Projection Neurons Mimics the Transcriptional Signature of Huntington’s Disease. Biomedicines 10 (2022). https://doi.org/10.3390/biomedicines10102377
- Tshilenge, K. T. et al. Proteomic Analysis of Huntington’s Disease Medium Spiny Neurons Identifies Alterations in Lipid Droplets. Mol Cell Proteomics 22, 100534 (2023). https://doi.org/10.1016/j.mcpro.2023.100534
- Gurkar, A. U. et al. Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat Aging 3, 776-790 (2023). https://doi.org/10.1038/s43587-023-00446-6
-
 Hao Cheng Bioinformatics Scientist
Hao Cheng Bioinformatics ScientistHao Cheng is a Bioinformatics Scientist with a master's degree in computer science. Hao develops skills in large-scale genomics data processing and analysis, involving statistical modeling, data visualization, machine learning, and in-house bioinformatics pipeline optimization. In addition, he had experience in deep learning and multi-omics analysis.
hcheng@buckinstitute.org
-
 Chad A. Lerner, PhD Postdoctoral Researcher
Chad A. Lerner, PhD Postdoctoral ResearcherChad Lerner received his PhD in molecular biology at the Drexel College of Medicine in 2013, and completed a T32 NIH training fellowship at the University of Rochester aimed at exploring the effect of electronic cigarette oxidants on a critical animal lung antioxidant defense system and in mitochondria. His earlier graduate studies revealed mitochondrial involvement in the conferred benefit of converging pathways between rapamycin and low IFG-1 signaling on lifespan. His duties include training the Institute’s technical and research staff for cell respirometry and advanced live-cell and high-content microscopy and to carry out such assays as service.
CLerner@buckinstitute.org
-
 Sima Taghizadeh Data Scientist
Sima Taghizadeh Data ScientistSima Taghizadeh is a Data Scientist with a Master's degree in electrical and computer engineering from the University of Iowa, specializing in image data analysis for biomedical research. With expertise in deep learning, Sima excels in developing strategies and algorithms for processing and understanding complex biomedical images. This includes managing large-scale data from automated microscopes and designing automated workflows to analyze this data efficiently. Sima's work primarily focuses on using advanced techniques like convolutional neural networks and morphological image processing methods to enhance data analysis in scientific projects.
STaghizadeh@buckinstitute.org
Associate Research Professor
Director of the Morphology and Imaging Core
AGerencser@buckinstitute.org
Commonly used ΔψM assays often lead to data misinterpretation, and this has been frequently ignored. The cause of misinterpretations is that all ΔψM probes are influenced by multiple properties of cells other than ΔψM, such as ΔψP, geometry and probe binding. These pitfalls have been described by us1,2 and by our colleague David Nicholls3 in detail. We have designed and applied a technology to calculate ΔψM by accounting for all identified factors influencing probe fluorescence in monolayer cell cultures, resulting in absolute magnitude of ΔψM in millivolts calibrated values4,5.
The ΔψM assay is based on recording fluorescence time courses of the cationic dye TMRM (tetramethylrhodamine methyl ester) together with an anionic, fluorescent plasma membrane potential indicator FLIPR (FLIPR plasma membrane potential kit; Molecular Devices). The principle of the assay is to create internal calibration points at the end of a time course recording, which in turn, are used for calculating baseline, and any other potentials in the recording1.
A detailed protocol for this assay1 is available here (open access): Unbiased Millivolts Assay of Mitochondrial Membrane Potential in Intact Cells
We also provide this technology as a service through the Buck’s Morphology and Imaging Core.
References:
- Lerner CA, Gerencser AA. Unbiased Millivolts Assay of Mitochondrial Membrane Potential in Intact Cells. Methods Mol Biol. 2022;2497:11-61. doi: 10.1007/978-1-0716-2309-1_2. PubMed PMID: 35771433; PMCID: PMC9377305.
- Gerencser AA, Brand MD. Exploiting Mitochondria In Vivo as Chemical Reaction Chambers Dependent on Membrane Potential. Molecular Cell. 2016;61(5):642-3. doi: 10.1016/j.molcel.2016.02.026. PubMed PMID: 26942666.
- Nicholls DG. Fluorescence measurement of mitochondrial membrane potential changes in cultured cells. In: Palmeira CM, Moreno AJ, editors. New York: Humana Press; 2012. p. 119-33.
- Gerencser AA, Mookerjee SA, Jastroch M, Brand MD. Measurement of the Absolute Magnitude and Time Courses of Mitochondrial Membrane Potential in Primary and Clonal Pancreatic Beta-Cells. PloS one. 2016;11(7):e0159199. Epub 20160712. doi: 10.1371/journal.pone.0159199. PubMed PMID: 27404273; PMCID: PMC4942067.
- Gerencser AA, Chinopoulos C, Birket MJ, Jastroch M, Vitelli C, Nicholls DG, Brand MD. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. The Journal of physiology. 2012;590(Pt 12):2845-71. Epub 20120410. doi: 10.1113/jphysiol.2012.228387. PubMed PMID: 22495585; PMCID: PMC3448152.
In collaboration with Shona Mookerjee, we have developed theoretical background and practical calculation worksheets to convert common readouts of the Seahorse Extracellular Flux Analyzer into pmoles of ATP produced per time by the sample, enabling a direct comparison of the two ATP producing pathways, glycolysis and mitochondria (including oxidative and substrate-level phosphorylation)1.
The technology1 is described here (open access): Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements.
The calculation of JATP requires the buffering power2 of the medium, as described here (open access): Measurement and Analysis of Extracellular Acid Production to Determine Glycolytic Rate.
The latest calculation Excel worksheets can be downloaded from GitHub.
We also provide this technology as a service through the Buck’s Morphology and Imaging Core.
References:
- Mookerjee SA, Gerencser AA, Nicholls DG, Brand MD. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. Journal of Biological Chemistry. 2017;292(17):7189-207. Epub 20170307. doi: 10.1074/jbc.M116.774471. PubMed PMID: 28270511; PMCID: PMC5409486.
- Mookerjee SA, Brand MD. Measurement and Analysis of Extracellular Acid Production to Determine Glycolytic Rate. Journal of Visualized Experiments. 2015;2(106):1-9. doi: 10.3791/53464. PubMed PMID: 26709455.
The harsh redox environment inside living cells, compounded with variables that affect intracellular probe accumulation, render intracellular detection of ROS imprecise, non-specific and misleading, regardless of what probe is used. While typical commercial intracellular ROS-probes respond well to exogeneous pro-oxidants, we have never observed proper responses with such probes to modulation of mitochondrial superoxide and H2O2 production. Therefore, we used the extracellular H2O2 detection system Amplex Red / horseradish peroxidase system in our studies. This detection system sensitively resolves variations in native mitochondrial ROS production.
Methods for using the Amplex Red / horseradish peroxidase system in monolayer cell cultures1,2 can be found here (open access):
- Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions
- Effects of sugars, fatty acids and amino acids on cytosolic and mitochondrial hydrogen peroxide release from liver cells
References:
- Gibbs ET, Lerner CA, Watson MA, Wong HS, Gerencser AA, Brand MD. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem J. 2023;480(5):363-84. doi: 10.1042/BCJ20220611. PubMed PMID: 36862427.
- Fang J, Zhang Y, Gerencser AA, Brand MD. Effects of sugars, fatty acids and amino acids on cytosolic and mitochondrial hydrogen peroxide release from liver cells. Free Radic Biol Med. 2022;188:92-102. Epub 20220616. doi: 10.1016/j.freeradbiomed.2022.06.225. PubMed PMID: 35716827.
The best resource for our Ca2+-imaging-related protocols are the methods sections of the following papers:
Calibration of single-wavelength Ca2+ probes4:
Calibration of ratiometric Ca2+ probes2,3:
- No Consistent Bioenergetic Defects in Presynaptic Nerve Terminals Isolated from Mouse Models of Alzheimer’s Disease
- Real-time visualization of cytoplasmic calpain activation and calcium deregulation in acute glutamate excitotoxicity
Mitochondrial Ca2+ using Rhod-2-AM or X-rhod-1-AM1,5:
- Selective, high-resolution fluorescence imaging of mitochondrial Ca2+ concentration
- Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell death and differentiation
Multiphoton microscopic Ca2+ imaging8:
Cytosolic and mitochondrial NADH/NAD+ using multiphoton microscopy6,7:
- Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions
- Mitochondrial dysfunction induces senescence with a distinct secretory phenotype
Also see protocols for analyzing such image data here:
References:
- Kushnareva YE, Gerencser AA, Bossy B, Ju WK, White aD, Waggoner J, Ellisman MH, Perkins G, Bossy-Wetzel E. Loss of OPA1 disturbs cellular calcium homeostasis and sensitizes for excitotoxicity. Cell death and differentiation. 2012;20(2):353-65. Epub 20121109. doi: 10.1038/cdd.2012.128. PubMed PMID: 23138851; PMCID: PMC3554330.
- Choi SW, Gerencser AA, Ng R, Flynn JM, Melov S, Danielson SR, Gibson BW, Nicholls DG, Bredesen DE, Brand MD. No Consistent Bioenergetic Defects in Presynaptic Nerve Terminals Isolated from Mouse Models of Alzheimer’s Disease. Journal of Neuroscience. 2012;32(47):16775-84. doi: 10.1523/JNEUROSCI.2414-12.2012. PubMed PMID: 23175831; PMCID: PMC3736741.
- Gerencser AA, Mark KA, Hubbard AE, Divakaruni AS, Mehrabian Z, Nicholls DG, Polster BM. Real-time visualization of cytoplasmic calpain activation and calcium deregulation in acute glutamate excitotoxicity. Journal of neurochemistry. 2009;110(3):990-1004. Epub 20090529. doi: 10.1111/j.1471-4159.2009.06194.x. PubMed PMID: 19493161; PMCID: PMC2745075.
- Gerencser AA, Adam-Vizi V. Mitochondrial Ca2+ dynamics reveals limited intramitochondrial Ca2+ diffusion. Biophysical journal. 2005;88(1):698-714. Epub 20041022. doi: 10.1529/biophysj.104.050062. PubMed PMID: 15501949; PMCID: PMC1305047.
- Gerencser AA, Adam-Vizi V. Selective, high-resolution fluorescence imaging of mitochondrial Ca2+ concentration. Cell calcium. 2001;30(5):311-21. doi: 10.1054/ceca.2001.0238. PubMed PMID: 11733937.
- Gibbs ET, Lerner CA, Watson MA, Wong HS, Gerencser AA, Brand MD. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem J. 2023;480(5):363-84. doi: 10.1042/BCJ20220611. PubMed PMID: 36862427.
- Wiley CD, Velarde MC, Lecot P, Liu S, Sarnoski EA, Freund A, Shirakawa K, Lim HW, Davis SS, Ramanathan A, Gerencser AA, Verdin E, Campisi J. Mitochondrial dysfunction induces senescence with a distinct secretory phenotype. Cell Metabolism. 2016;23(2):303-14. Epub 20151210. doi: 10.1016/j.cmet.2015.11.011 , eprint = 9605103. PubMed PMID: 26686024; PMCID: PMC4749409.
- Deng H, Gerencser AA, Jasper H. Signal integration by Ca 2+ regulates intestinal stem-cell activity. Nature. 2015;528(7581):212-7. Epub 20151202. doi: 10.1038/nature16170. PubMed PMID: 26633624; PMCID: PMC4669953.
Microscopy image analysis nearly always requires customization. This is because of the combined effects of microscopy modality, probes or staining, and biology that involves differing specimens and treatment conditions. Thus, there is no one-size-fits all; therefore Dr. Gerencser has developed an image processing platform, Image Analyst MKII, for fast prototyping of fully customizable image analysis pipelines.
Specific protocol using such an analysis pipeline can be found here: Image Analyst MKII Protocols.
This software, and related technology has been used in over 60 scientific publications.
Selected Publications
- Gurkar, A. U. et al. Spatial mapping of cellular senescence: emerging challenges and opportunities. Nat Aging 3, 776-790 (2023). https://doi.org/10.1038/s43587-023-00446-6
- Gibbs, E. T. et al. Site IQ in mitochondrial complex I generates S1QEL-sensitive superoxide/hydrogen peroxide in both the reverse and forward reactions. Biochem J 480, 363-384 (2023). https://doi.org/10.1042/BCJ20220611
- Lerner, C. A. & Gerencser, A. A. Unbiased Millivolts Assay of Mitochondrial Membrane Potential in Intact Cells. Methods Mol Biol 2497, 11-61 (2022). https://doi.org/10.1007/978-1-0716-2309-1_2
- Gerencser, A. A. Metabolic activation-driven mitochondrial hyperpolarization predicts insulin secretion in human pancreatic beta-cells. Biochimica et Biophysica Acta (BBA) – Bioenergetics 1859, 817-828 (2018). https://doi.org/10.1016/j.bbabio.2018.06.006
- Mookerjee, S. A., Gerencser, A. A., Nicholls, D. G. & Brand, M. D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. Journal of Biological Chemistry 292, 7189-7207 (2017). https://doi.org/10.1074/jbc.M116.774471
- Gerencser, A. A., Mookerjee, S. A., Jastroch, M. & Brand, M. D. Positive Feedback Amplifies the Response of Mitochondrial Membrane Potential to Glucose Concentration in Clonal Pancreatic Beta Cells. Biochimica et Biophysica Acta – Molecular Basis of Disease 1863, 1054-1065 (2017). https://doi.org/10.1016/j.bbadis.2016.10.015
- Brand, M. D. et al. Suppressors of Superoxide-H2O2 Production at Site IQ of Mitochondrial Complex I Protect against Stem Cell Hyperplasia and Ischemia-Reperfusion Injury. Cell Metab 24, 582-592 (2016). https://doi.org/10.1016/j.cmet.2016.08.012
- Gerencser, A. A. Bioenergetic Analysis of Single Pancreatic β-Cells Indicates an Impaired Metabolic Signature in Type 2 Diabetic Subjects. Endocrinology 156, 3496-3503 (2015). https://doi.org/10.1210/en.2015-1552
- Gerencser, A. A. et al. Quantitative measurement of mitochondrial membrane potential in cultured cells: calcium-induced de- and hyperpolarization of neuronal mitochondria. The Journal of physiology 590, 2845-2871 (2012). https://doi.org/10.1113/jphysiol.2012.228387
- Gerencser, A. A. et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Analytical chemistry 81, 6868-6878 (2009). https://doi.org/10.1021/ac900881z
- Gerencser, A. A. & Adam-Vizi, V. Selective, high-resolution fluorescence imaging of mitochondrial Ca2+ concentration. Cell calcium 30, 311-321 (2001). https://doi.org/10.1054/ceca.2001.0238
Dr. Gerencser’s full publications list


