by Buck Institute
April 24, 2023 . News
Three years after a global shut down, Buck CEO Eric Verdin weighs in on Covid-19 and the aging immune system
 The death toll from Covid-19 is nearing nearly 7 million worldwide. Even though the virus appears here to stay, most people have resumed their normal lives, thanks to a barrier of immunity built from infections and vaccines. But the virus continues to spread, and variants still pose a threat. In addition to further understanding the virus itself, research is also focused on developing treatments that block infection and/or bolster the body’s response to it. Buck President and CEO, Eric Verdin, MD says understanding aging needs to be a key factor in the effort. He discussed all of this in a conversation with Kris Rebillot, Buck’s senior director of communications.
The death toll from Covid-19 is nearing nearly 7 million worldwide. Even though the virus appears here to stay, most people have resumed their normal lives, thanks to a barrier of immunity built from infections and vaccines. But the virus continues to spread, and variants still pose a threat. In addition to further understanding the virus itself, research is also focused on developing treatments that block infection and/or bolster the body’s response to it. Buck President and CEO, Eric Verdin, MD says understanding aging needs to be a key factor in the effort. He discussed all of this in a conversation with Kris Rebillot, Buck’s senior director of communications.
Eric, I’ve heard you describe Covid-19 as a disease of aging. Why?
Just take a look at this chart from the Centers for Disease Control and Prevention, the CDC.
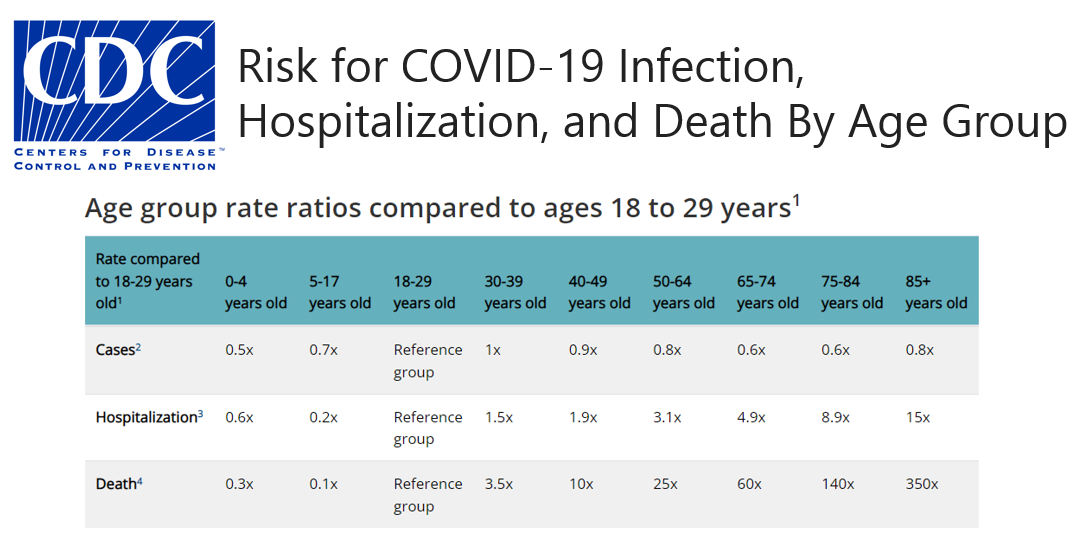 The figures are pretty stark. While the virus infects all people regardless of age, your age is a key risk factor for hospitalizations and death starting at 30. The fact that those over 85 are 350 more times likely to die from Covid is an eye opener. Even those 65 to 74, which can be a healthy cohort, are 60 times more likely to succumb to the disease. That’s why I say that understanding aging needs to be included in the research mix of our effort to combat Covid-19.
The figures are pretty stark. While the virus infects all people regardless of age, your age is a key risk factor for hospitalizations and death starting at 30. The fact that those over 85 are 350 more times likely to die from Covid is an eye opener. Even those 65 to 74, which can be a healthy cohort, are 60 times more likely to succumb to the disease. That’s why I say that understanding aging needs to be included in the research mix of our effort to combat Covid-19.
It’s obvious that many older people are not able to mount an effective immune response to the infection, especially before we had a vaccine. Let’s go back to the basics. What happens to the immune system with aging? And what do we know about how that impacted the response to Covid-19?
We have two immune systems. Our first responder is the innate immune system. It’s non- specific, and it has no memory. It kicks in when the body first detects a pathogen and is supposed to provide a first protective barrier against infection. But as we age, the innate immune system often becomes chronically activated, a condition responsible for the chronic inflammation of aging or “inflammaging.” Two of the reasons for this are the accumulation of damage, like misfolded proteins, and a process called cellular senescence, which results in the secretion of inflammatory cytokines. Epigenetic regulation, which influences the production of proteins in our cells, can also get disturbed during aging. During Covid-19 there was clear evidence that the inflamed innate immune system lacked the precise required regulation; it went into continuous overdrive, resulting in what has been coined a “cytokine storm” which caused multi-organ failure and death.
The adaptive immune system is very different; it’s specific and is activated by exposure to pathogens using immunological memory to learn about the threat and respond accordingly. Every infection you’ve had during your life primes the adaptive immune system. Imagine adaptive immunity like an army that has been trained to recognize a specific enemy. You can have an army, but nobody knows how to fight. They haven’t been taught. But imagine that the army has gone through one war. They come back, they’re organized, they know how to trigger the right response to the threat.
It can take several days to fully activate the adaptive immunity following an infection. The adaptive immune system includes both T cells and B cells. T cells can destroy infected or cancerous cells. They also direct the immune response by helping B lymphocytes to eliminate invading pathogens. Here’s one area where age comes into play - it’s called thymic involution. The thymus is a gland that is behind your sternum and in front of your heart. It is the place where your T-cells go to become “educated” to recognize specific pathogens, and to amplify and divide. This is where they’re generated. The thymus is one of the first organs to involute during aging and we don’t fully understand why or how this happens. By the time you’re 50, most of the thymus, like the ovary, is gone. There is a lot of research going on in the aging field trying to understand thymic involution. Thymic involution is the reason why your T cell response decreases with aging. This decreases your ability to mount an effective immune response during vaccination and to respond appropriately during an infection.
I’ve heard you talk about vaccines and the fact that in 30% - 40% of people over 70 the vaccine doesn’t work. That’s because of thymic involution?
Yes, this is a large part of the problem.
That’s depressing. What can we do?
First of all, it’s important for everyone, no matter their age, to get vaccinated. It’s safe and it adds to our collective protection. There’s also evidence that vaccinations help prevent long Covid.
Focusing on vaccinations gives me an opportunity to talk about lifestyle choices which have a huge impact on our immune health, and in some cases, might even boost the efficacy of vaccines.
 Let’s start with regular moderate exercise. It promotes circulation in our lymph system. It gets our T cells moving. It dramatically reduces the systemic inflammation we talked about earlier. Exercise also reduces the risk of upper respiratory tract infections. A study from Iowa State University shows that 90 minutes of exercise immediately after vaccination increases long-term antibody response to both the flu and Covid-19 vaccines. That’s really impressive. One caveat here: this study involved light-to- moderate-intensity exercise. There’s evidence that prolonged high intensity exercise coupled with the stress of competition can depress the immune system. Elite athletes often report an increase in respiratory infections in the days following competition.
Let’s start with regular moderate exercise. It promotes circulation in our lymph system. It gets our T cells moving. It dramatically reduces the systemic inflammation we talked about earlier. Exercise also reduces the risk of upper respiratory tract infections. A study from Iowa State University shows that 90 minutes of exercise immediately after vaccination increases long-term antibody response to both the flu and Covid-19 vaccines. That’s really impressive. One caveat here: this study involved light-to- moderate-intensity exercise. There’s evidence that prolonged high intensity exercise coupled with the stress of competition can depress the immune system. Elite athletes often report an increase in respiratory infections in the days following competition.

Another thing you can do to protect your immune system and boost the effectiveness of vaccines is to make sure you get enough sleep in the days surrounding vaccination. Poor sleep wreaks havoc on both the innate and adaptive immune system. A study from the University of Chicago shows that getting less than six hours of sleep blunts antibody response to both the flu and Covid-19 vaccinations. When it comes to Covid-19 vaccines, this research shows that the effects of insufficient sleep are the equivalent to two months of waning antibodies after vaccination.
 Before we delve into nutrition let me say that numerous aging mechanisms, biological pathways that impact how we age, have an enormous impact on our immune system. This is complex science and looking at nutrition in terms of aging and immunity is probably one of the most complex and we do not fully understand the mechanisms at play. That being said, it is clear that eating unhealthy foods stimulates pro-aging pathways, including pathways involving inflammation and immunity.
Before we delve into nutrition let me say that numerous aging mechanisms, biological pathways that impact how we age, have an enormous impact on our immune system. This is complex science and looking at nutrition in terms of aging and immunity is probably one of the most complex and we do not fully understand the mechanisms at play. That being said, it is clear that eating unhealthy foods stimulates pro-aging pathways, including pathways involving inflammation and immunity.
There are a large number of micronutrients, such as vitamins and minerals, as well as macronutrients including some amino acids, fatty acids and cholesterol, that exert very important and specific impacts on appropriate immune activity. This study goes into some depth in regard to Vitamins A, B1, B2, B3, B12, C and D, along with zinc and selenium as influencers of both innate and adaptive immunity. Here’s the catch, while some of them have anti-inflammatory effects, they may also have pro-inflammatory effects, depending on one’s biological make up and interactions with other nutrients. That’s why I encourage everyone to avoid supplements and to get their vitamins and minerals from food. Overdosing on a particular vitamin or mineral is almost impossible with a whole food diet while there is clear evidence that large amounts of specific vitamins can also have deleterious effects.
The jury is still out when it comes to boosting immunity through intermittent fasting, which is very popular among researchers studying aging. This observational study involving long-term intermittent fasters showed a lower severity of Covid-19 symptoms in those infected prior to the availability of vaccines. While limiting food intake has been shown to decrease inflammatory markers in both humans and mice, another study in mice shows that skipping breakfast may not be good for immunity. My take? Extreme fasting is likely detrimental to health. If you’re interested in intermittent fasting, start slow and monitor how your body responds.
 Finally, let’s talk about social relationships. They are very important to immune health. This study identifies two distinct immune pathways that link social relationships with health. Social adversity, such as social isolation or loneliness, is associated with increased inflammation, while social contact is linked to a better immune response to viruses. Humans are deeply social and thrive on social interactions. In addition, there is growing evidence that living under adverse conditions, such as poverty, racism, long-term exposure to pollution, and threats of violence not only damage our immune system but make for shorter lifespans. These are issues that research on aging is just beginning to grapple with.
Finally, let’s talk about social relationships. They are very important to immune health. This study identifies two distinct immune pathways that link social relationships with health. Social adversity, such as social isolation or loneliness, is associated with increased inflammation, while social contact is linked to a better immune response to viruses. Humans are deeply social and thrive on social interactions. In addition, there is growing evidence that living under adverse conditions, such as poverty, racism, long-term exposure to pollution, and threats of violence not only damage our immune system but make for shorter lifespans. These are issues that research on aging is just beginning to grapple with.
Coming out of the pandemic, what excites you about the science?
Well first, I’m thrilled that it’s much safer for us to interact with each other! We recently had our first public event at the Buck in three years; it was wonderful to welcome members of our community back into our beautiful space on Mount Burdell.
And, secondly, I’m really excited about the revolutionary mRNA technology that allowed for vaccines to be developed in such a short amount of time. The effectiveness of the vaccines has really highlighted the fact that there is a lot of potential for novel vaccines against everything—cancer, malaria, and sickle-cell anemia among many others. And I know of dozens of clinical trials from other companies that are currently rushing in to take advantage of this new technology.
And as a physician trained in immunology who is now focused on aging, I am very pleased to see an increased focus on how aging impacts the immune system and our responses to viruses such as Covid-19, the Flu and RSV. Given the close link between aging and immune pathways we have multiple opportunities to intervene. Everything we are working on, whether it is to prevent heart disease or macular degeneration, is likely to prevent you from dying from a virus as well. To me, it is an amazing finding. And I am really excited about a big paper that is currently in pre-print (meaning it’s not been peer-reviewed). It shows that treatment with metformin, a drug for diabetes that impacts many aging pathways, protects against long-Covid when given within four days of symptom onset. That is a big “wow” in my universe. So, stay tuned. I think that as awful as the pandemic has been, there is a silver lining for research on aging. We intend to take full advantage of it.
Science is showing that while chronological aging is inevitable, biological aging is malleable. There's a part of it that you can fight, and we are getting closer and closer to winning that fight.
Eric Verdin, MD, Buck Institute President and CEO