by Buck Institute
April 15, 2021 . BLOG
A Shot Towards Ending a Pandemic: Vaccines Explained
This is the 4th and final blog in our series from MS Biology students from Dominican University. In this post, student authors Taira Saracco, Joseph Morris, Fadzai Teramayi, & Elena Battistoni explain the science and development process of vaccines . You can catch up on the series by reading the first blog, about vaccines, the 2nd about prevention, and the third, about the history of pandemics.
By: Taira Saracco, Joseph Morris, Fadzai Teramayi, & Elena Battistoni;
Edited by Professor, Dr. Pankaj Kapahi
As vaccine availability begins to expand, it might be your turn to schedule an appointment, but before you do you might have some questions. What are COVID vaccines? Are they safe? Wasn’t the development of them rushed? Why should I get it? Every question is a good question, and the more they are asked and answered, the more we will understand how a vaccine can end a pandemic.
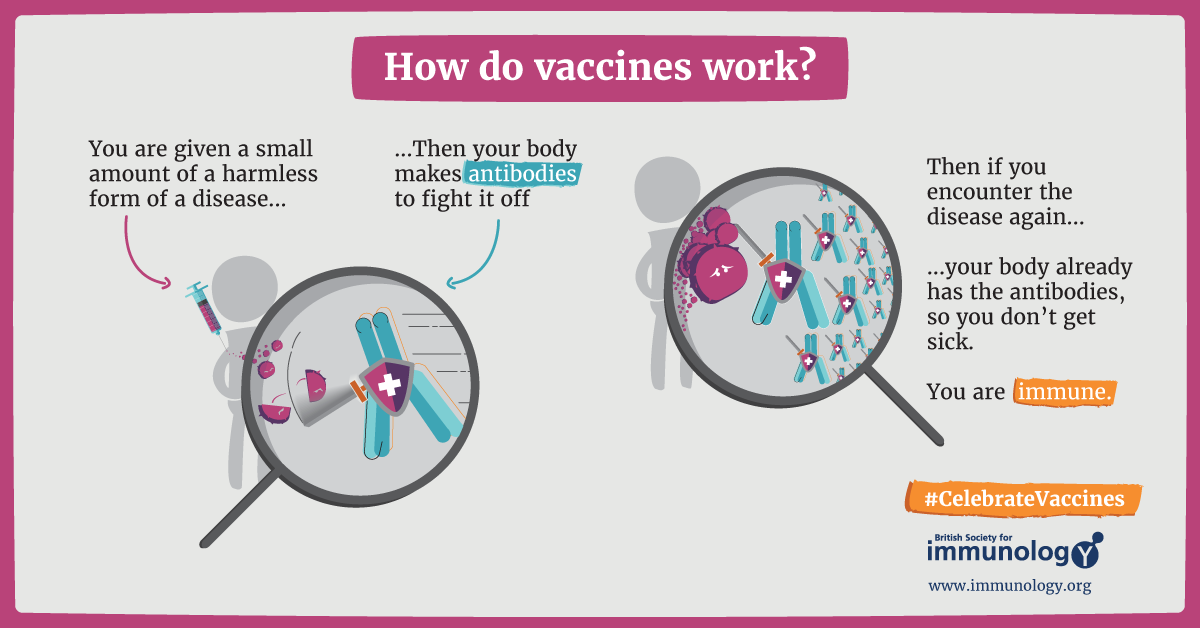
How Does a Vaccine Give Me Immunity?
Vaccines are administered to a person to create an immune response to a specific disease, in this case, the SARS-CoV-2 virus. The way a vaccine works can sound complicated but try to imagine it like a battle. A virus is trying to invade your body and you have no immunity. With no immunity, your body is not protected or prepared for battle from the “enemy”. However, with a vaccine, your body recognizes the “enemy” and sets up “soldiers” to fight - and you are able to avoid getting defeated! In this case, the “enemy” is the the virus causing the disease and the “soldiers” are your body’s antibodies, fighting off the virus and preventing you from getting sick.
What Are the Different Kinds of Vaccines?
There are four main types of vaccines (summarized in Table 1). Two of them are most familiar to us: the virus vaccines like the flu vaccine and the measles/mumps vaccine; and the protein-based vaccines like the whooping cough/tetanus vaccine. These vaccines have been around since the mid-20th century. The two other types of vaccines, the viral vector and mRNA vaccine, were more recently developed in the 1970s-1980s and are getting more publicity due to the current pandemic. By now, you’ve probably heard of at least one of the COVID-19 vaccines being distributed where you live, and the goal of this blog is to inform you about what these vaccines are, how they work, and the process of how they were developed.
Table 1: Different types of vaccines and how they work
|
Type of vaccine |
Virus |
Protein-Based |
Viral Vector |
mRNA |
|
What is it? |
Inactivated: Inactivated virus from the disease administered to body to induce immune response (flu/polio vaccine) Live-Attenuated: Living, but weakened version of virus administered for immune response (measles, mumps and rubella vaccine/chicken pox and shingles vaccine) |
Uses specific parts of the virus that the body recognizes as foreign to create an immune response (whooping cough/tetanus vaccines) |
Uses a different, harmless virus to introduce genetic material of SARS-CoV-2 virus into patient to induce immune response |
Uses mRNA of SARS-CoV-2 virus to give our body instructions on how to target foreign “spike protein” and build antibodies against it |
How Safe and Effective are Vaccines?
To this day, the question of the safety and efficacy of vaccines lingers in the media and the general public. Similar to any medicine, vaccines often have side effects. These side effects are usually mild and dissipate in a few days without the need for treatment. Common side effects include injection site reactions, fever, muscle aches, headache and fatigue. However, though extremely rare, there is still the possibility of a severe allergic reaction occurring. When we say rare, we mean that out of a million people receiving a vaccine, one or two people may have a severe reaction (CDC). In addition to safety, vaccine efficacy is another crucial parameter to take into account (HHS). Vaccine efficacy refers to vaccine protection in optimal conditions, usually with healthy participants in a random clinical trial. It is calculated by measuring the frequency of illness in vaccinated compared to unvaccinated groups. For example, in the Pfizer COVID-19 clinical trial, a common misconception is that 95% of vaccinated individuals are protected from the disease. Rather, a 95% vaccine efficacy means that the infection rate for vaccinated people is 95% lower than unvaccinated individuals. So, if 1% of unvaccinated people became infected, the rate for vaccinated people is only .05% (thelancelet).
Is COVID just like the flu? Why Do I Need a Vaccine?
In the early days of the COVID-19 pandemic, some information was contradictory, whether it was from the media, scientific community or even the government. This helped to lead to the sprouting of various misperceptions and even conspiracy theories. One of the leading misperceptions was that COVID-19 was just like the flu. Understandably, this drew in part from the knowledge that the method of transmission and some of the signs/symptoms of the diseases mirrored those of the flu. That is, both diseases are transmitted through respiratory droplets and signs and symptoms included fever, fatigue and headaches. However, this is not the full picture due to the difference in severity these diseases have. COVID-19 is relentlessly contagious and managed to overwhelm the US health system. To put numbers to it, on average, weekly death rates from COVID-19 were ~20 times higher than those of the flu during the peak seasons in 2018-2019 (Faust & Rio, 2020).
So with the detrimental effects of COVID, how is one to prevent getting it? Guidelines established by the CDC include wearing masks, social distancing and avoiding large gatherings. Recently, the development of COVID-19 vaccines provides an even better option for preventing the spread of the disease. Similarly, the most recommended form of prevention for the flu are traditional flu shots. It is interesting to note that this past year flu infection rates are historically low in the US with interseasonal levels of 0.2% vs the typical 1-2% (CDC).
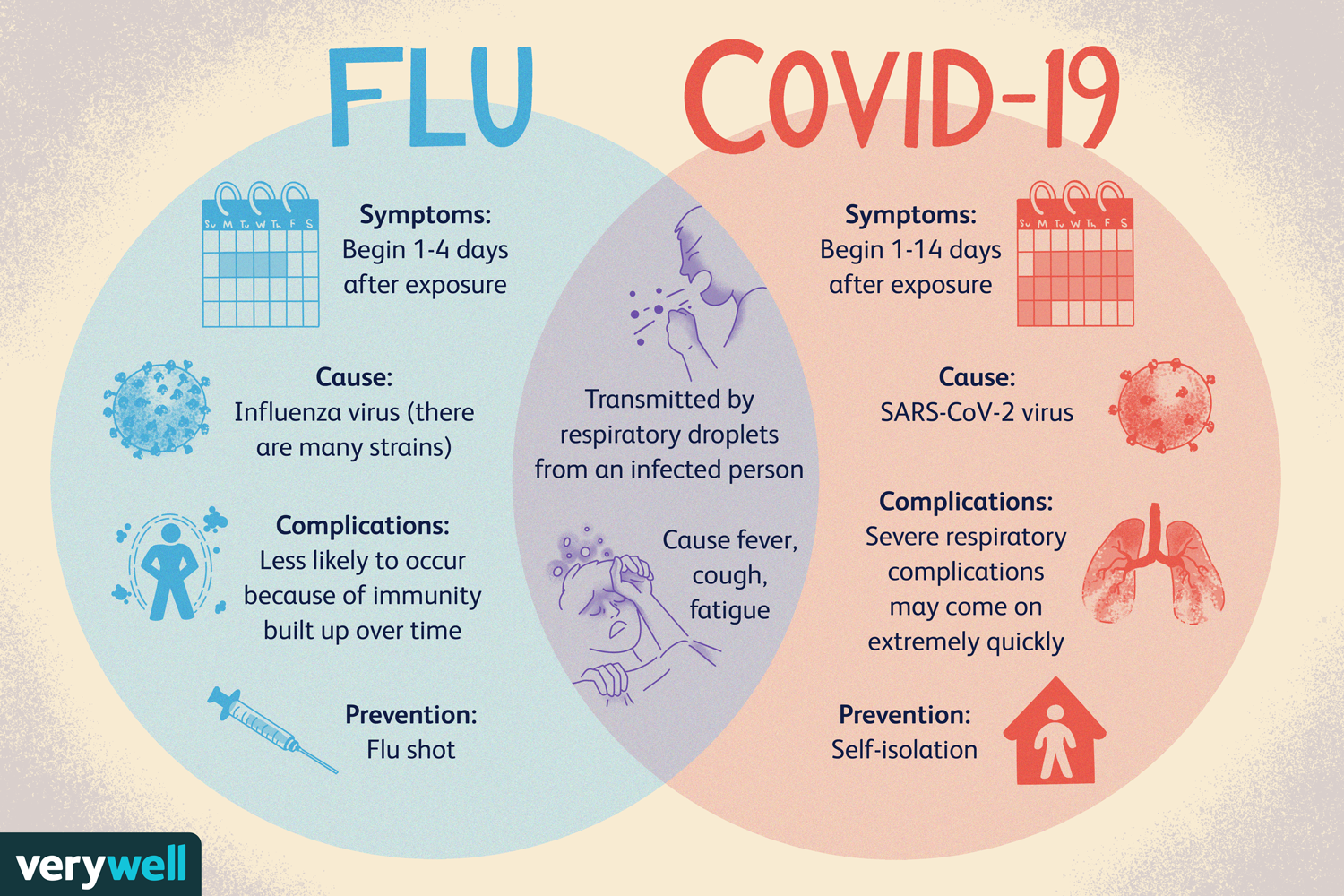
Source: Very Well Health: Flu Vs. Covid-19 https://www.verywellhealth.com/coronavirus-flu-differences-4798752
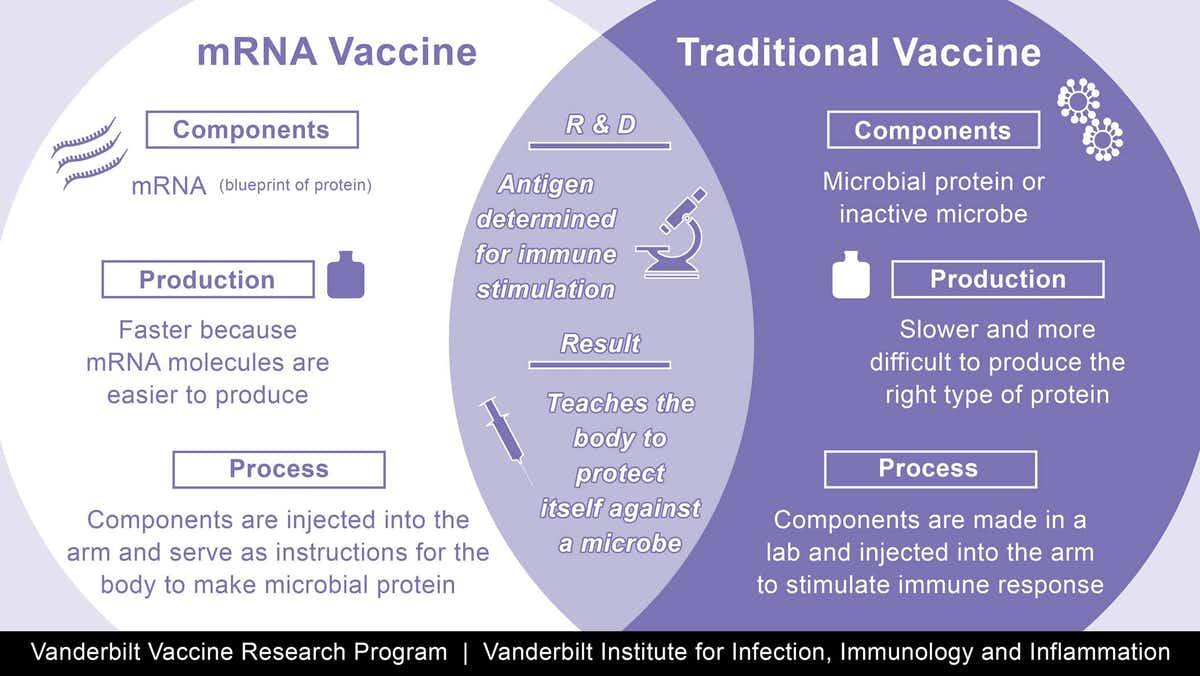
Between the Flu and Covid: How Has Vaccine Technology Improved?
Throughout our lives, there is a recommended vaccination schedule within each age group. This includes everything from the Hepatitis B vaccine at birth to the Tdap at age 65 and above. There are also seasonal vaccines recommended each year, as is the case with the flu shot. For additional details on the recommended vaccination schedule, you can refer to the CDC guide here. There are multiple ways these vaccines are manufactured. Typically, the process is expensive, lengthy and (in some cases) involves a year-round effort of data collection before the actual production. Conventional vaccine production carries a risk of incomplete activation in cases where a live attenuated flu virus is used to make the vaccine. As a result, there has been research advancement in mRNA vaccines. The anticipated advantages of an mRNA vaccine include the ability to be rapidly modified and manufactured. In addition, the mRNA vaccine is expected to help the body respond more efficiently as well as not burden the internal and chemical conditions of the host's body. These advantages are exemplified by the mRNA COVID-19 vaccines from Moderna and Pfizer.
So Just How Do the COVID-19 Vaccines Work?
Worldwide, the vaccines approved for providing COVID-19 immunity use all four main approaches for providing acquired immunity for vaccinated individuals that were described above.
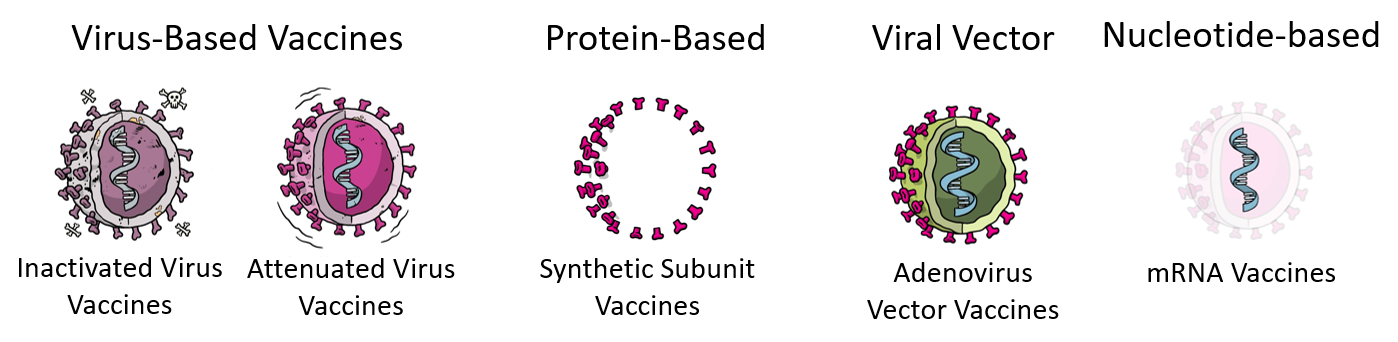
While there are a number of approved COVID-19 vaccines that use traditional vaccine technologies that have been used in previous vaccines such as those for the seasonal flu, polio, hepatitis A, and rabies, COVID-19 has allowed the biomedical community to fast track the development of several new approaches, namely peptide-based, adenovirus-based, and mRNA-based vaccines.
Although viral vector vaccines have had a long history of being explored, such as the vaccine for Ebola, the adenovirus vectors, which are responsible for the common cold, were not employed until the COVID-19 pandemic. The adenovirus vector COVID-19 vaccine recently approved for emergency use in the USA is the Johnson & Johnson vaccine, AstraZeneca and Oxford vaccine is currently undergoing testing and approval in the USA. In addition to these adenovirus vector vaccines, during this pandemic the first synthetic peptide-based vaccine by the Vector institute was developed, which uses part of the spike protein on COVID-19 to create an immune response.
And what about those mRNA vaccines that have received so much attention? While COVID-19 mRNA vaccines from Moderna and Pfizer are the first ever approved using this technology mRNA itself is nothing new as a possible vaccination strategy. In fact, research related to mRNA vaccines have decades of history in the scientific literature, spanning back to 1989 (in this paper) when RNA was delivered to cells by lipids, similar molecules used in the two mRNA COVID-19 vaccines. Since then, significant strides have been made using mRNA as potential vaccines, which have been validated as not only safe, but also remarkably effective in creating immunity for viruses like COVID-19. For more details on the vaccines approved for COVID-19, see Table 2 below.
So, which vaccine should I get?
A common question many of you may have is that, with all these vaccines to choose from, which one should I get? The bottom line: get the one that is available to you. Don’t get too caught up in efficacy numbers or how many shots are required. The best thing to do to help move our population closer to herd immunity is to simply get what is available to you. All of them provide excellent protection and the faster we reach herd immunity, the faster we can return to a more normal (hopefully better!) way of life.
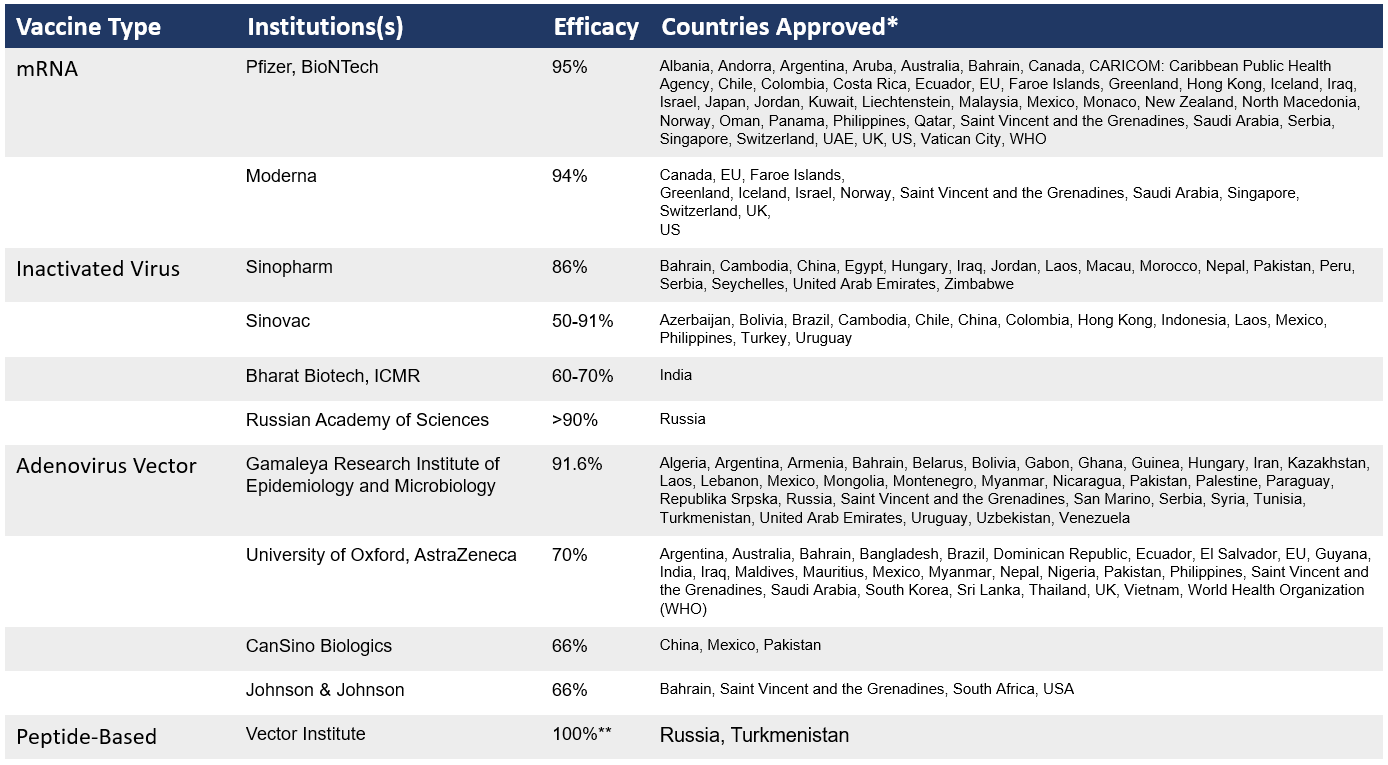
*As of March 1, 2021. **According to Russian sanitary watchdog; public data unavailable. For more information see: https://www.verywellhealth.com/coronavirus-flu-differences-4798752
COVID-19 unified scientists worldwide to innovate and accelerate: How the COVID-19 vaccines were approved so quickly.
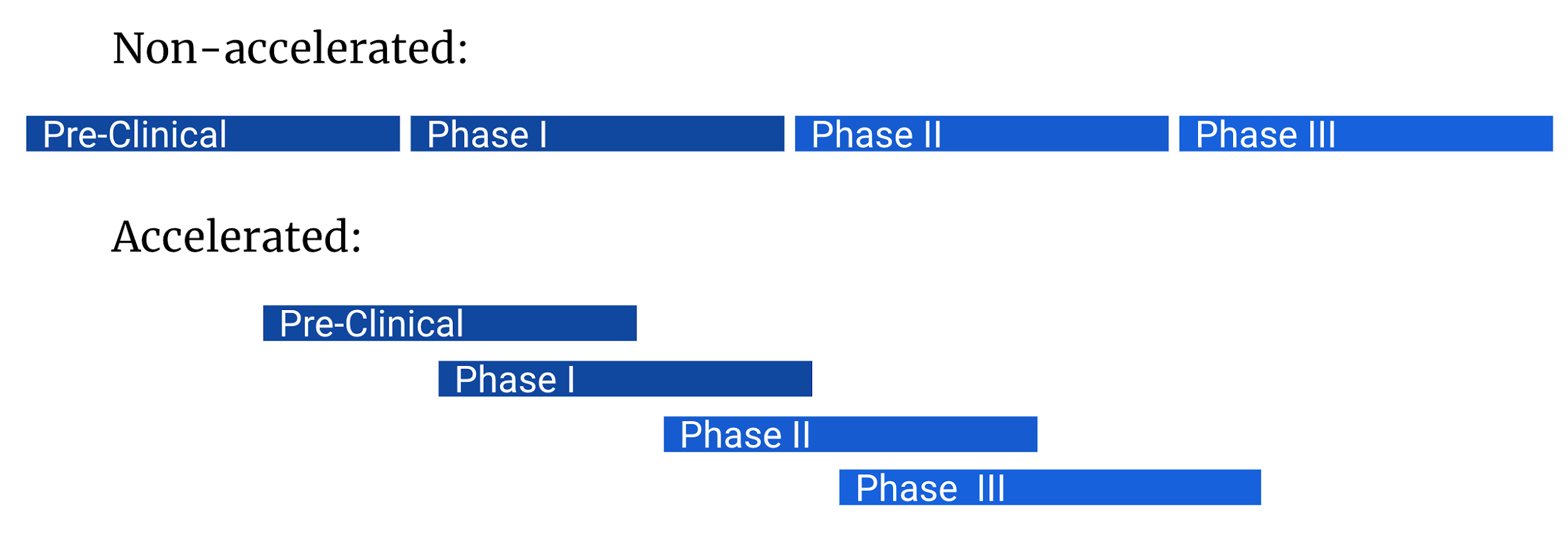
The COVID-19 pandemic has unified efforts from the global biomedical community to accelerate vaccine development using both traditional vaccine technologies as well as burgeoning new technologies. Since lockdowns first began in the US during March 2020, over a dozen vaccines have been approved for use in countries across the world, as shown below in the timeline. Three of these have already received FDA emergency use authorization in the U.S. As of publication, the Johnson & Johnson vaccine is currently on pause, but is expected to be back in use shortly following additional safety analysis. Such rapid development of this impressive array of vaccines that utilize a variety of strategies to combat the SARS-CoV-2 virus highlights how a united scientific community can rapidly find solutions to even very difficult problems. Despite vaccines being developed extremely quickly, no safety considerations were cut out of the process, which is further detailed in the following sections.

Timeline of COVID-19 vaccines approved for use in countries worldwide.
How were COVID-19 Vaccines Made?: The Journey of A Potential Vaccine
Vaccines, like the Pfizer and Moderna COVID-19 vaccines, are types of drugs and must therefore undergo multiple assessments to determine safety and efficacy before being distributed. There are three main agencies of the U.S. Department of Health and Human Services (HHS) you may have heard of during the COVID-19 pandemic:
- S. Food and Drug Administration (FDA), which has a Center for Biologics Evaluation and Research (CBER) that is in charge of regulating vaccines
- The Center for Disease Control and Prevention (CDC), which has an Advisory Committee on Immunization Practices (ACIP)
- Vaccine Adverse Event Reporting System (VAERS), a national system used by scientists at FDA and the CDC to collect reports of side effects that happen after vaccination
Together, the above agencies ensure that vaccines provided to the public have evidence of being safe and effective. The following is a basic outline of how a candidate vaccine goes from development by scientists in a research lab to being distributed for widespread use.
From Development to Distribution
Step 1: In the Exploratory Stage, basic laboratory research is done to identify natural or synthetic antigens (i.e. molecules that bind to antibodies and cause an immune response) that might help prevent the disease.
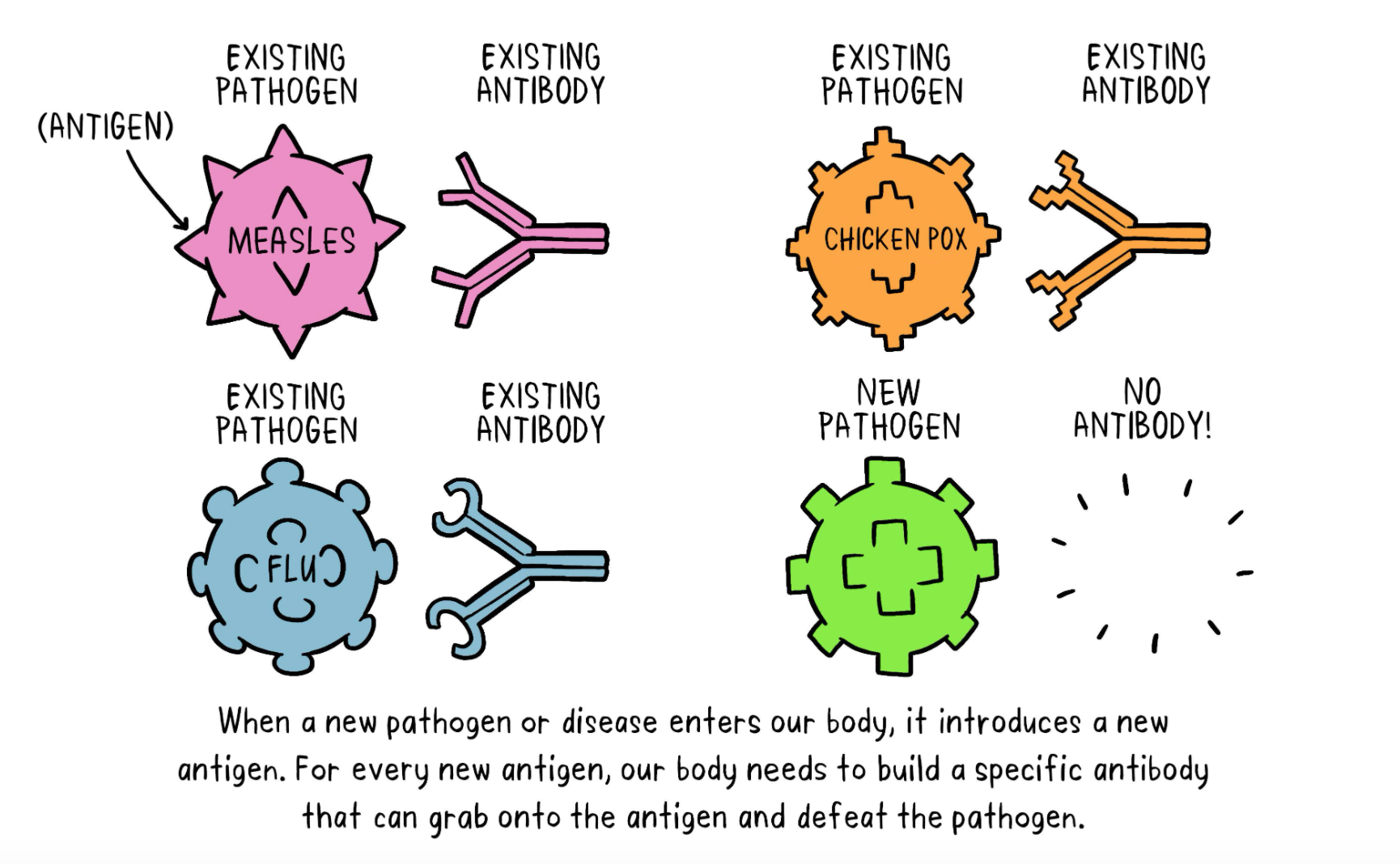
Source: World Health Organization: How Do Vaccines WorK? https://www.who.int/news-room/feature-stories/detail/how-do-vaccines-work
Q: How is the exploratory stage different during the COVID-19 pandemic?
A: Many of the laboratories that are studying COVID-19 provided open access to their data on the mechanisms of the virus. This facilitated international collaboration between researchers and accelerating the process of developing a vaccine that can illicit an immune response against the virus.
Explore more of the data and literature: National Center for Biotechnology Information: SARS-CoV-2 Resources
In addition, both national and international programs were designed to fund and coordinate accelerated development. Some examples of these programs include the following:
- National Institute of Health’s (NIH) Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), described as a “public-private partnership to develop a coordinated research strategy for prioritizing and speeding development of the most promising treatments and vaccines” (National Institute of Health ACTIV)
- The World Health Organization (WHO) created the the R&D Blueprint in order to “accelerate diagnostics, vaccines and therapeutics for this novel coronavirus” (World Health Organization R&D Blueprint Covid-19)
Step 2: After laboratory research, Pre-Clinical Trials test the identified antigens in cell-culture, tissue-culture, and animals to assess the safety of the candidate vaccine and its immunogenicity (aka, its ability to provoke an immune response)
Step 3: If the data from pre-clinical trials shows potential benefit of the candidate vaccine, its developers, referred to as sponsors, will submit an Investigational New Drug Application (IND) to the FDA’s Center for Biologics Evaluation and Research (CBER)
- The IND includes a description of the manufacturing and testing processes, a summary of laboratory reports, and details of the proposed study
Step 4: If the FDA approves the IND, the next stage is Clinical Trials With Human Subjects, which takes place in multiple phases:
- Phase I involves a small number of healthy adults (20-50 people)
- Phase II involves 100s of people, including those with varying health statuses and across different demographics
- Phase III involves thousands of people, often across multiple sites and multiple countries
Q: How are the pre-clinical and clinical trials different during the COVID-19 pandemic?
A: In standard vaccine development, trials are run in chronological order, with manufacturing only ramping up during the later stages of clinical trials with human subjects. In response to a public health emergency, trials run simultaneously with manufacturing scale-up occurring at earlier stages.
Step 5: If the data from the clinical trials show that the candidate vaccine shows beneficial effects, the sponsor will submit a New Drug Application (NDA) to the FDA:
-
- The NDA contains all of the data gathered to date about the vaccine. A typical NDA consists of at least 100,000 pages!
Learn more at: World Health Organization: Manufacturing, safety and quality control of vaccines https://www.who.int/news-room/feature-stories/detail/manufacturing-safety-and-quality-control
Q: How is the FDA approval process different during the COVID-19 pandemic?
A: After a public health emergency has been declared, sponsors can submit an application for the use of a drug they believe will act as a medical countermeasure (MCM). There is still a rigorous assessment by multiple regulatory bodies that determine whether an MCM can be approved. See below for this process.

Infographic Source: What Are Medical Counter Measures? - FDA.gov https://www.fda.gov/emergency-preparedness-and-response/about-mcmi/what-are-medical-countermeasures#infographic
Several COVID-19 vaccines have been issued Emergency Use Authorizations (EUAs), which allowed for their use to prevent COVID-19 under the specific circumstances. These circumstances are the existence of a public health emergency that has the potential to do significant harm and the lack of adequate, approved, or available alternatives for treatment or prevention.
Step 6: If the vaccine is approved by the FDA, the CDC’s Advisory Committee on Immunization Practices (ACIP) reviews all the data, votes whether to recommend the drug to the public, and what to do when supplies are limited.
It’s important to note that even after a vaccine is approved, it is still being monitored after distribution to catch any adverse side effects that may only be discernible with widespread use. That’s where the Vaccine Adverse Event Reporting System (VAERS) comes in. Any person can report side effects to VAERS, who will determine if there is a significant correlation between a specific vaccine and adverse side effects.
How Effective is Vaccination at Mitigating Outbreaks?
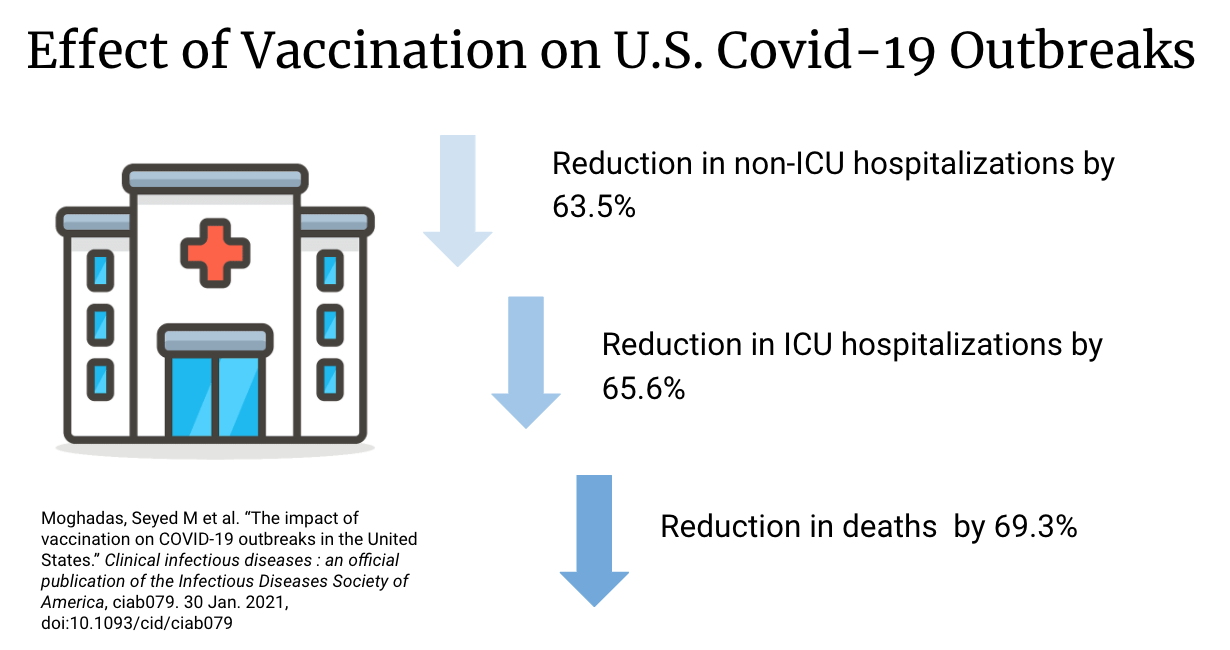
In a paper published in January 2021, Seyed M et al described the effect of vaccination in the United States. In their study, vaccination reduced the overall attack rate to 4.6% from 9.0% without vaccination over 300 days. The highest relative reduction in overall attack rate was observed among individuals aged 65 and older. Vaccination also significantly reduced adverse outcomes, with non-ICU hospitalizations, ICU hospitalizations, and deaths decreasing by 63.5%, 65.6%, and 69.3%, respectively, across the same period. Their results indicate that vaccination can have a substantial impact on mitigating COVID-19 outbreaks. However, the researchers did want to note that the adherence to non-pharmaceutical interventions is essential to achieve this impact. These other interventions include as mask wearing, contact-tracing, and isolation of infected cases.
COVID-19 Vaccine FAQs:
Will the vaccine give me COVID-19?
No, the vaccines do not use a live virus from SARS-CoV-2. They contain genetic information that create the spike protein responsible for COVID-19. Your body recognizes this foreign protein and activates an immune response.
I received my vaccines, but now I’m feeling side effects - am I sick?
After receiving the vaccine, you may feel certain side effects (pain/swelling near the injection site, headache, body aches, fever, and tiredness) which is normal. These are signs your body is having an immune response, but do not mean you are sick with COVID.
Wasn’t the development of the vaccine rushed?
COVID-19 vaccine development was not rushed. All clinical trials and safety protocols required by the FDA were completed in parallel to expedite the process.
Do I still need to wear a mask and maintain social distancing after being vaccinated?
Yes, continue to protect yourself and others. The CDC is still researching if the vaccines prevent us from spreading COVID-19, so it is still necessary to follow these guidelines to stop the spread and the pandemic.
Should I still get vaccinated if I previously had COVID-19?
Yes, experts still don’t know exactly how long you are protected from reinfection after recovering from COVID-19. It is possible, although rare, to become infected again with COVID-19 so the vaccine will give you more protection.
How long does the vaccine protect me from COVID-19?
Research is still being done to know how long the vaccine will protect you. However, if you get the vaccine and become infected with COVID-19, the disease will be less deadly than if you were infected without the vaccine.
Can I get the vaccine if I am pregnant or breastfeeding?
There is no evidence that the antibodies formed from the vaccine will harm the baby or the development of the placenta while pregnant or breastfeeding.
What are the long term effects of the vaccine?
Research is still ongoing when it comes to its effects in the long term. Candidates for the trial of vaccines from a manufacturer like Pfizer will continually be observed for the next couple of years.
Click here for more COVID-19 vaccine FAQs and the new CDC guidelines for fully vaccinated individuals.
Read More & Stay Updated
U.S. Food and Drug Administration Covid-19
Center for Disease Control and Prevention Covid-19
World Health Organization Covid-19
The Different Kinds of Covid-19 Vaccines
Covid-19 Clinical Trial Data

SHARE