by Buck Institute
January 26, 2021 . BLOG
Healing a broken heart: Time, or decreasing expression of a single protein
By Dominican University Graduate Students, Michael Broussalian, Lithgow lab,
and Asia Davis-Castillo, Melov lab; Edited by Dr. Pankaj Kapahi, Buck Professor

 The heart is generally considered to be a terminally differentiated organ, meaning that heart cells, also known as cardiomyocytes, lose their ability to divide during infancy. Since adult cardiomyocytes are unable to reenter the cell cycle, hearts are unable to regenerate tissue after injury, like heart attack or even age-related declines. Similar to a cut on the skin, an injury to the heart leads to scarring, but the scar does not disappear through regeneration of cells. These scars can lead to irregular heartbeat, heart failure, and death. There are currently no treatments that can effectively replace or regrow the heart tissue that is lost during injury.
The heart is generally considered to be a terminally differentiated organ, meaning that heart cells, also known as cardiomyocytes, lose their ability to divide during infancy. Since adult cardiomyocytes are unable to reenter the cell cycle, hearts are unable to regenerate tissue after injury, like heart attack or even age-related declines. Similar to a cut on the skin, an injury to the heart leads to scarring, but the scar does not disappear through regeneration of cells. These scars can lead to irregular heartbeat, heart failure, and death. There are currently no treatments that can effectively replace or regrow the heart tissue that is lost during injury.
A team from Tongji University School of Medicine in Shanghai, China consulted the literature and identified a protein that is essential for heart growth in the early stages of life: low-density lipoprotein receptor-related protein 6 (LRP6). LRP6 plays a key role in a pathway critical for early development. It had previously been shown to be essential for heart development; however, little was known about its role in cardiomyocyte growth. The team investigated the role of LRP6 in heart regeneration by proving that decreasing the amount of this protein leads to increased cell cycle activity, improves overall heart function, and reduces scarring in mice after injury. You read that right: even though this protein is important for heart cell growth in developing animals, decreasing LRP6 in older individuals can actually lead to more cell division. The team safely and successfully reduced the amount of LRP6 in human stem cell-derived cardiomyocytes as an in-vitro model for the human heart, thus indicating the potential for a treatment in humans.
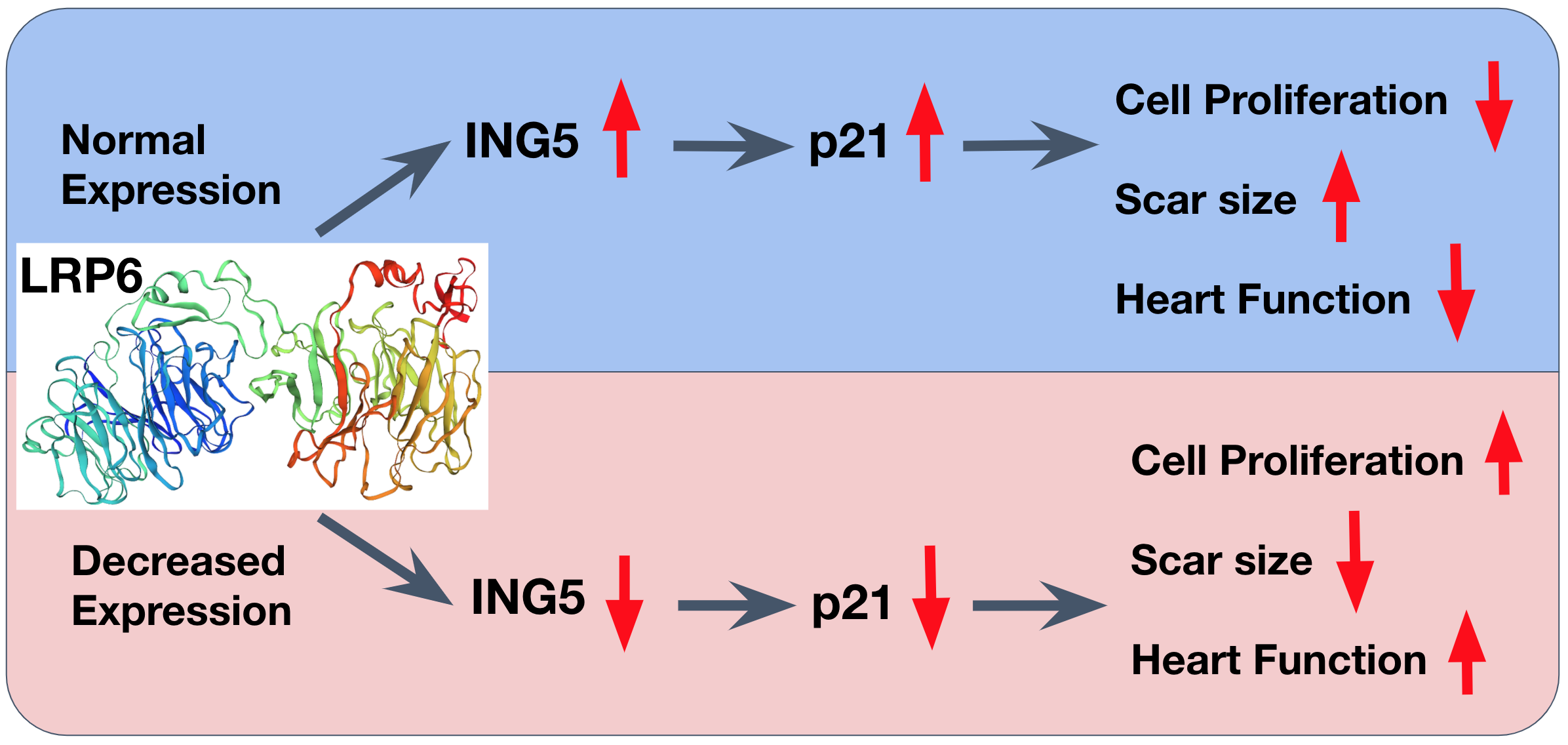
Figure 1. Proposed pathway of LRP6 regulation of cardiomyocyte proliferation. Normal expression of LRP6 causes cell cycle arrest early in life via the ING5/p21 pathway. Viral injection of LRP6 miRNA or Tamoxifen treatment causes LRP6 downregulation, leading to a decrease in ING5/p21 activity. Wu et al. find that this decrease in expression is sufficient to induce cell proliferation, reduce scar size, and improve heart function after injury.
Further scientific investigation is still required to confirm the safety of intervention in injured hearts that are still functioning in living subjects. The Tongji University team also raised the idea that small molecules and synthetic molecules that target LRP6 specifically could be a more favorable option for treatments.
In addition, this model of successful cell-cycle reentry, in which cells start dividing again, could be used to answer other questions pertaining to age-related pathologies, such as whether inducing cell cycle reentry will cause decreased senescence in cardiomyocytes. Senescent cells accumulate with age and contribute to the normal aging process as well as age-related disorders. To test this, scientists could block production of LRP6 in mice and then measure senescence markers in every few weeks until death. The senescence marker protein p21 would be particularly interesting to use here, as this study found that p21 decreases when LRP6 is reduced. In theory, the resulting decrease in p21 activity could diminish senescence in the heart, thus potentially delaying senescence-induced age-associated physical decline. This research is substantial for the scientific community because it shows that manipulating expression of LRP6 could lead to the development of new treatments to promote heart regeneration and repair.
LRP6 downregulation promotes cardiomyocyte proliferation and heart regeneration
Yahan Wu, Liping Zhou, Hongyu Liu, Ran Duan, Huixing Zhou, Fulei Zhang, Xiaoyu He, Dongbo Lu, Ke Xiong, Maolin Xiong, Jinzhu Zhuang, Yi Liu, Li Li, Dandan Liang & Yi-Han Chen
Cell Research, 1-13 (2020)

SHARE