by Buck Institute
December 6, 2018 . BLOG
A perennial molecule that treats pellagra and pauses aging
By Geoff Meyerhof, Dominican University Master's Student in the Kapahi lab
The ancient process of "nixtamalization," or boiling corn in an alkaline solution prior to consumption, helped pre-Columbian Mesoamericans extract proteins and vitamins from otherwise nutrient-sparse corn. By doing this, these tribes were able to largely avoid disease arising from nicotinamide adenine dinucleotide (NAD+) deficiency. Recently, researchers from Ecole Polytechnique Fédérale de Lausanne (Katsyuba et al., 2018) have published in Nature a modern method to enhance our NAD+ levels.
In this latest iteration, Katysuba et al. have shown there is an evolutionarily conserved approach to enhance production of NAD+, a coenzyme that improves health and extends lifespan.
These researchers focused on a novel enzymatic target, ACSMD, that when inhibited, assists the body with de novo NAD+ synthesis. Although this group is the first to inhibit ACSMD for the purpose of bolstering NAD+ and lifespan, they are far from the first to demonstrate the overall positive effects of NAD+ on health.
In fact, Pellagra, a disease stemming from NAD+ deficiency, has been studied for over 200 years. In the mid-18th century, long before the connection between vitamin B3 and NAD+ was known, the Spanish physician, Gaspar Casal, postulated a link between a dietary deficiency and Pellagra. However, a causal relationship between a nutrient-poor diet and the four D's of Pellagra — dermatitis, diarrhea, dementia, and death — was not firmly established until the early 1900's. Dr. Joseph Goldberger, the Surgeon General of the United States, published findings in 1917 that demonstrated that symptoms of Pellagra could be reversed via protein supplementation. In the spirit of Dr. Goldberger, Katsyuba et al. supplemented the diet of worms with the NAD+ precursor tryptophan, which in turn extended their lifespan.
NAD+ acts as a redox coenzyme for reactions involved in metabolism and as a co-substrate for several enzymes that regulate gene expression. Given these dual roles, NAD+ can be thought of as a sensor that links a cell's energy level to gene expression. The body can synthesize NAD+ using either vitamin B3 as precursors (via the "Preiss-Handler" or "salvage" pathway) or from the amino acid, tryptophan (Fig. 1). However, as an organism grows older, its levels of NAD+ decrease. Hence the question arises, if you increase your NAD+ levels, do you live longer? Indeed, there are several lines of evidence that suggest that this is the case. Researchers have shown that enhancing NAD+ through either genetic or dietary interventions, extends both healthspan (the healthy years of life) and lifespan in multiple model organisms.
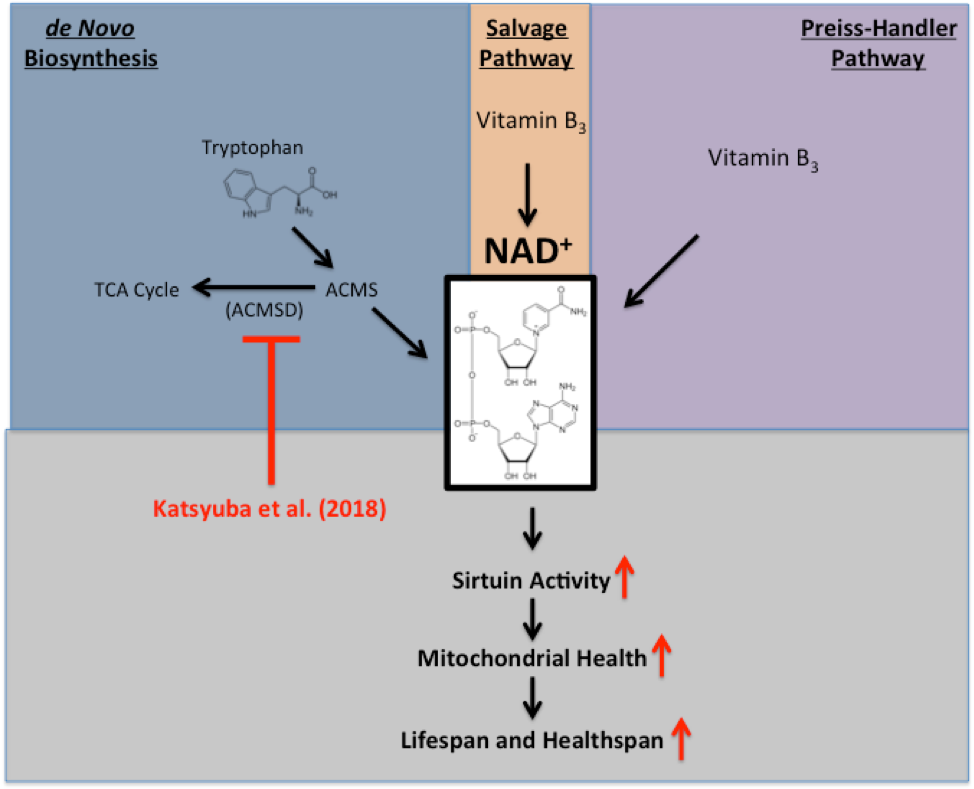
Figure 1. Pathways of NAD+ Biosynthesis and the Consequences of ACMSD Inhibition. NAD+ can be synthesized from three independent pathways, the Preiss-Handler pathway, the salvage pathway, and the de novo biosynthesis pathway. The Preiss-Handler pathway and salvage pathway synthesize NAD+ from dietary vitamin B3 (nicotinamide, nicotinic acid, and nicotinamide riboside). The de novo biosynthesis pathway synthesizes NAD+ from dietary tryptophan. To enhance NAD+ synthesis, Katsyuba et al. bolster the de novo pathway by inhibiting the enzyme ACMSD. They find this enhances sirtuin activity, improves mitochondrial health, and increases lifespan and healthspan.
Why is this? One paradigm suggests that lifespan extension from NAD+ is inextricably linked with the NAD-dependent enzymes, Sirtuins, and mitochondrial health. Namely, it appears that NAD+ enhances Sirtuin activity, which influences gene expression and helps to induce the "mitochondrial unfolded protein response". By inducing the mitochondrial unfolded protein response, the mitochondria mount a de facto garbage cleaning effort that helps maintain their ability to power the cell with age.
Equipped with this knowledge, Katsyuba et al. set out to find a way to help the body produce more NAD+ on its own (i.e. de novo NAD+ synthesis). To accomplish this, researchers focused on the enzyme, aminocarboxymuconate semialdehyde decarboxylase (ACMSD). Unlike the pronunciation of its full name, the role of ACMSD in NAD+ synthesis is very simple. It can be summarized as follows: when we eat protein, the body can convert the amino acid tryptophan into NAD+ through an intermediate called ACMS. But, the enzyme ACMSD also "uses" ACMS. Therefore, when there is more ACMSD present, less ACMS gets converted into NAD+. So naturally, the authors aimed to inhibit ACMSD (Fig. 1). Fortunately, the researchers found two drugs that do just that.
Katsyuba et al. discovered that worms, mice, and human cells with reduced ACMSD activity (achieved either by genetic or pharmacological means) all produce more NAD+ and have improved mitochondrial health. Excitingly, blocking ACMSD in worms even extended their mean lifespan by up to 18%. Leaving no stone unturned, the authors of this paper also tested the effect of anti-ACMSD drug treatment on two mouse models of disease: a non-alcoholic fatty liver disease model and an acute kidney injury model. When these mice were fed ACMSD-inhibiting drugs, each showed improvements in their respective disease phenotypes.
In 1937, two years after the death of Dr. Joseph Goldberger, nicotinic acid was identified as a potent anti-Pellagra agent. When a dog with Pellagra was fed nicotinic acid, not only did its dermatitis resolve, but it also began to grow again. In light of the data presented by Katsyuba et al., it is tempting to speculate that this stunted growth was the result of an energy imbalance stemming from mitochondrial dysfunction. Whatever the case may be, it is now clear that NAD+ does much more than prevent acute disease. Katsyuba et al. have thoroughly characterized a novel approach to augment NAD+ levels and demonstrated its significance to health and aging. It will be exciting to see whether these pro- NAD+ compounds also extend life and healthspan in mammals, and of course, how they perform in the clinic.
SHARE