by Buck Institute
January 8, 2019 . BLOG
Running trails or genetic pathways: The future of exercise
By G. W. Brownridge III and Nicole L. McIntosh, Dominican University Master's Students
What if you could receive the benefits of exercise moving only the muscles necessary to take a pill? The alluring concept of effortless fitness has motivated scientists seeking the elusive “exercise pill” for years, but a study published last summer in the journal Cell Metabolism indicates this enticing dream may soon become reality. The study gained widespread popularity when Ron Evans and his team at the Salk Institute successfully boosted the athletic endurance of mice by 70% using a drug named GW501516 (GW for short). Designed to represent the average American, mice used in the study lived a sedentary lifestyle with a diet comprised nearly entirely of fat and sugar. While mice in the control group grew overweight and lethargic, those given daily doses of GW remained lean and energized, despite their unhealthy lifestyle. Furthermore, mice given GW ran roughly 100 minutes longer during treadmill tests than their “couch potato” counterparts, earning them the nickname “Lance Armstrong Mice.”
How did Lance Armstrong Mice stay fit without exercise or a healthy diet? The key to their Armstrong-esque vitality lies in activation of a specific genetic pathway. Triggered by natural exercise, this PPAR delta (PPARδ) pathway plays a major role in fatty acid metabolism. The PPARδ gene behaves as a metabolic switch in muscles. When activated, it sets off a cascade of biochemical changes enabling muscles to shift from using carbohydrates to burning fat for energy. GW works by binding to the PPAR delta receptor, essentially “flipping” the switch to boost the breakdown of fat and stop the breakdown of carbohydrates. In turn, preservation of carbohydrates gives Lance Armstrong Mice greater endurance by slowing the rate at which their muscles deplete glucose stores. By chemically activating the PPAR delta pathway with GW, Evans’ team demonstrated muscle metabolism can be altered as it is with exercise sans any actual physical effort. In this way, GW acts as an “exercise mimetic” creating the same metabolic shifts chemically as exercise does mechanically (Figure 1). As Evans puts it, “100 minutes is a huge increase in performance for sedentary mice that never actually trained…It would take a lot of diligent training every single day to get that benefit, and these mice are getting it just because we’re feeding them a drug that is reprogramming their metabolic properties.”
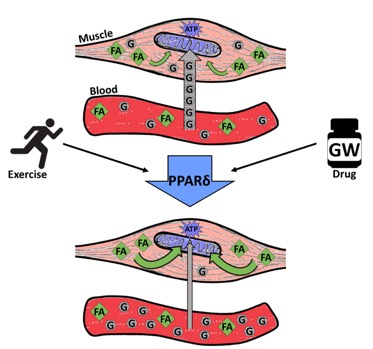
Figure 1. Graphical Abstract of Exercise- or Drug-Induced PPARδ-Mediated Metabolic Shift in Muscle. When activated via exercise or drug (GW), PPARδ acts as a metabolic switch to shift muscles from using carbohydrates (glucose, G, depicted in grey) to burning fat (fatty acids, FA, depicted in green) for energy. This metabolic shift spares blood sugar, effectively slowing the rate at which mice reach exhaustion during treadmill tests. Credit: Nicole McIntosh
So, should we trade in our favorite pair of trainers for a pill box? The history of GW suggests not. Developed in the late ‘90s by researchers at GlaxoSmithKline (GSK), GW initially gave promising results in preclinical trials with obese monkeys, not only decreasing their fat but improving their cholesterol and insulin sensitivity as well. Grooming GW as treatment for “metabolic syndrome,” a combination of precursor symptoms to heart disease and diabetes that more than a third of Americans exhibit, GSK boasted encouraging results through Phase II clinical trials in humans. However, in 2007, just prior to entering Phase III, GSK dropped GW trials when long-term toxicity-test results revealed mice given GW for two years (a lifetime for them) developed tumors in multiple organs throughout their bodies.
Though the toxicity-test results devastated the GSK trials, they did not discourage everyone. Almost as soon as GSK researchers dropped GW, Evans’s team picked it up, publishing a study just a year later demonstrating the positive effects of GW coupled to exercise training on mouse endurance. Despite safety risks, GW quickly hit the black market, landing on The World Anti-Doping Agency’s (WADA’s) list of banned substances in 2009. Still, use of GW outside of the research lab continued, prompting WADA to issue a rare warning to cheating athletes in 2013 of the possible health hazard associated with GW. Though low doses of GW in humans may not be toxic, testing this would require a 70-year human trial. Evans is choosing a faster route, focusing his team on developing a less potent version of GW in hopes that it will be less toxic. Until this hope is supported by a long-term toxicity study, these authors are choosing to keep their gym memberships.
Arguably more interesting than the quest to develop the exercise pill is the thought of what exactly having an exercise pill would mean. Evans’s team is not the only one studying an “exercise mimetic,” and as questioned by Australian protein biologist David James, “what are you going to mimic?” Indeed, while a preponderance of studies link exercise to good health, the biochemical changes induced by exercise are still relatively unknown. Without knowing exactly why, the broad range of health benefits attributed to exercise span anywhere from stronger bones to ease in learning new languages.
Will there ever be a pill able to replicate such far-reaching, diverse implications as exercise? Theodore Garland, a UC Riverside biologist studying physical activity across species, would rather see a pill that increases our motivation to exercise. Such a pill might ease the qualms of those concerned that drugs inducing a vast metabolic change in place of exercise may create tumors and be detrimental to health.
As with all drugs, the cost-to-benefit ratio will need to be weighed. For the average adults looking to regain their youthful physique, potential benefits may not outweigh the risks. Alternatively, for a patient suffering from Duchenne Muscular Dystrophy, a genetic disease causing muscle breakdown and inevitable death in 1 in 5000 boys, the risk may be entirely worth it. It is this disease Evans is seeking FDA approval in treating with his modified less-potent version of GW. He is optimistic that his and other exercise mimetics will be approved for legal consumption in humans in the next decade or so. While critics of these drugs worry they will undermine the value of exercise, perhaps the most insightful point in this discussion was provided by Nicola Twilley who covered this story for the New Yorker. Rather than simply undermine exercise, she points out that “Evans’s research may redefine exercise—for better and for worse—in much the same way that other fields of metabolic research have gradually redefined food…A morning jog will be reclassified as a good source of beneficial chemicals; sports may be redesigned to optimize their molecular outcomes.”
At any rate, there is time for those on both sides to formulate opinions and continue this important discussion, with Evans’s or any other exercise pill multiple years and trials away from appearing in our medicine cabinets.

SHARE